Chitosan-heparin nano-particles and cell matrix-removed biomaterial processed by using the same
A nanoparticle and biomaterial technology, which is applied in anticoagulant treatment, packaging item types, special packaging items, etc., can solve the problems that the replacement speed and degree of endothelial cells and tissues cannot meet the ideal requirements, and achieve long-term sustainable curative effect , Improving biocompatibility and stabilizing particle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of chitosan-heparin nanoparticles: improve the ion cross-linking method, the molecular weight of chitosan is controlled between 100kD-300kD, the concentration of chitosan solution is 1-4mg / ml, the heparin is low molecular weight heparin and unfractionated heparin, the concentration is 0.5 -2mg / ml, the temperature of the solution system is: 37°C, using a medium-speed magnetic stirring method, and the time is 20 minutes.
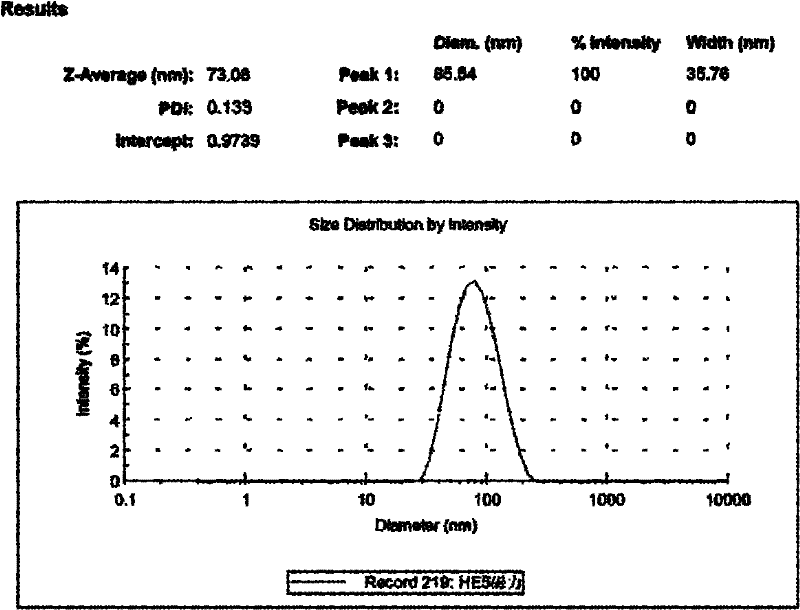
[0041]Results: By controlling the factors related to the preparation of nanoparticles, the particle size of chitosan-heparin nanoparticles was controlled between 50nm and 200nm, the range of PDI was between 0.110 and 0.185, and the Zata potential was between -20mv and +30mv. Nanoparticles can form a good core-shell structure with negative charges on the outermost layer according to needs, and the stable release time can reach more than 60 days after being further reinforced by EDC / NHS. (See figure 1 )
[0042] Conclusion: Chitosan-heparin ...
Embodiment 2
[0044] (1) Bovine jugular vein decellularization-dye-mediated photooxidation treatment: the patent: used for pulmonary artery vascular repair or intermediate biological valve pipeline and its preparation method (patent number: ZL 200510032124.9) for decellularization and dye-mediated oxidation React in two steps.
[0045] (2) Nanoparticles are combined with bovine jugular veins through a two-step method. First, the nanoparticles and bovine jugular veins are physically adsorbed and self-assembled, and then chemically cross-linked by EDC / NHS: the bovine jugular veins are placed in 2 mg / ml nano In the particle solution, the self-assembly reaction time is 6 hours under the condition of a shaking speed of 80 rpm, and then chemical cross-linking and cross-linking are carried out for 4 hours to improve the bonding firmness of the nanoparticles and the material. EDC concentration is 200mmol / L, NHS concentration is 60mmol / L.
[0046] RESULTS: The nanoparticles were homogeneous and bou...
Embodiment 3
[0049] VEGF binds to the nanoparticle-loaded bovine jugular vein obtained in Example 2. A 50-200ng / ml VEGF PBS solution was prepared, and the bovine jugular vein loaded with nanoparticles was placed in the VEGF solution, and reacted below 25° C. for 4 hours. Locally fixed VEGF was detected by immunohistochemical method, and the remaining VEGF content in the solution was detected by ELISA method.
[0050] Results: VEGF could specifically bind to heparin in bovine jugular vein loaded with nanoparticles.
[0051] Conclusion: VEGF can be locally immobilized and slowly released by nano drug loading.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com