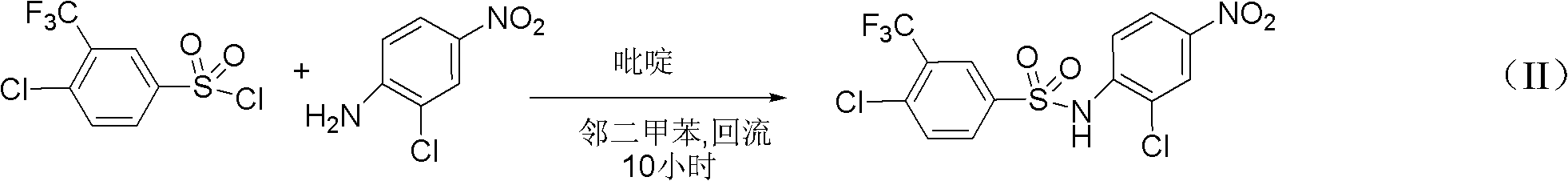
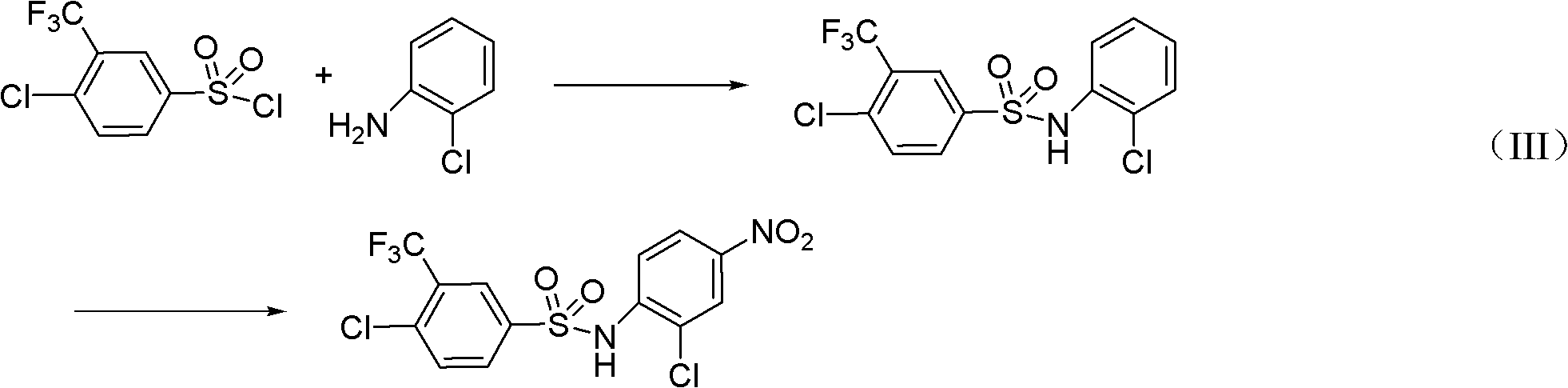
Preparation method for N-(2- chlorine-4-phenyl)-4- chlorine-3-trifluoromethyl benzene sulfonamide
A technology of trifluoromethylbenzenesulfonamide and trifluoromethylbenzenesulfonyl chloride is applied in the field of preparation of N--4-chloro-3-trifluoromethylbenzenesulfonamide, which can solve the problem of high reaction temperature and production safety There are many hidden dangers, not being used, etc., to achieve the effect of increasing the reaction yield, reducing production risks, and improving the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1.27 kilograms of o-chloroaniline and 100 grams of 4-dimethylaminopyridine were dissolved in 10 L of toluene, and the temperature was controlled at 50° C., and 2.87 kilograms of 4-chloro-3-trifluoromethylbenzenesulfonyl chloride was added dropwise. After the addition, 50 °C for 5 hours. Cooling, filtration, the filtrate recovered 8L of toluene, cooling, filtration, and ethanol recrystallization to obtain 2.52 kg of N-(2-chloro-4-phenyl)-4-chloro-3-trifluoromethylbenzenesulfonamide, yield 68 %.
Embodiment 2
[0031] 1.27 kg of o-chloroaniline and 100 g of 4-dimethylaminopyridine were dissolved in 6L of 1,2-dichloroethane, the temperature was controlled at 50°C, and 2.87 kg of 4-chloro-3-trifluoromethylbenzenesulfonyl chloride was added dropwise , After the dropwise addition was completed, the reaction was carried out at 50° C. for 5 hours. Cool, recover 4.5L of 1,2-dichloroethane, cool, filter, and recrystallize from ethanol to obtain N-(2-chloro-4-phenyl)-4-chloro-3-trifluoromethylbenzenesulfonamide 3.18 kg, yield 86%.
Embodiment 3
[0033] 1.27 kilograms of o-chloroaniline and 100 grams of 4-dimethylaminopyridine were dissolved in 14L of chlorobenzene, and the temperature was controlled at 50° C., and 2.87 kilograms of 4-chloro-3-trifluoromethylbenzenesulfonyl chloride was added dropwise. After the dropwise addition, React at 50°C for 5 hours. Cooling, filtration, filtrate reclaims 11L of chlorobenzene, cooling, filtration, ethanol recrystallization, obtains 2.66 kilograms of N-(2-chloro-4-phenyl)-4-chloro-3-trifluoromethylbenzenesulfonamide, yield 72%.
[0034] The second part: the preparation of sulfanil
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com