Application of polypeptides to preparation of medicament for treating or preventing rheumatoid arthritis
A rheumatoid and arthritis technology, applied in the field of polypeptides for the treatment or prevention of rheumatoid arthritis, can solve problems such as not taking too long, failing to improve the condition, and undisclosed treatment effects of other diseases, and achieve significant rheumatoid arthritis inflammation, significant social value and market value, and the effect of treating rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Polypeptide I, polypeptide II and polypeptide III were all synthesized by solid phase synthesis, purified by high performance liquid chromatography, and the purity of the polypeptide was determined by RP-HPLC. The synthesis method refers to patents 201110194918.0 and 201110370529.9.
[0048] Results: The purity identification results of the synthesized polypeptides were analyzed by reverse liquid chromatography as follows: the purity of polypeptide I, polypeptide II and polypeptide III were 96.94%, 99.30%, and 96.34%, respectively, and the purity was greater than 95%, meeting the design requirements.
Embodiment 2
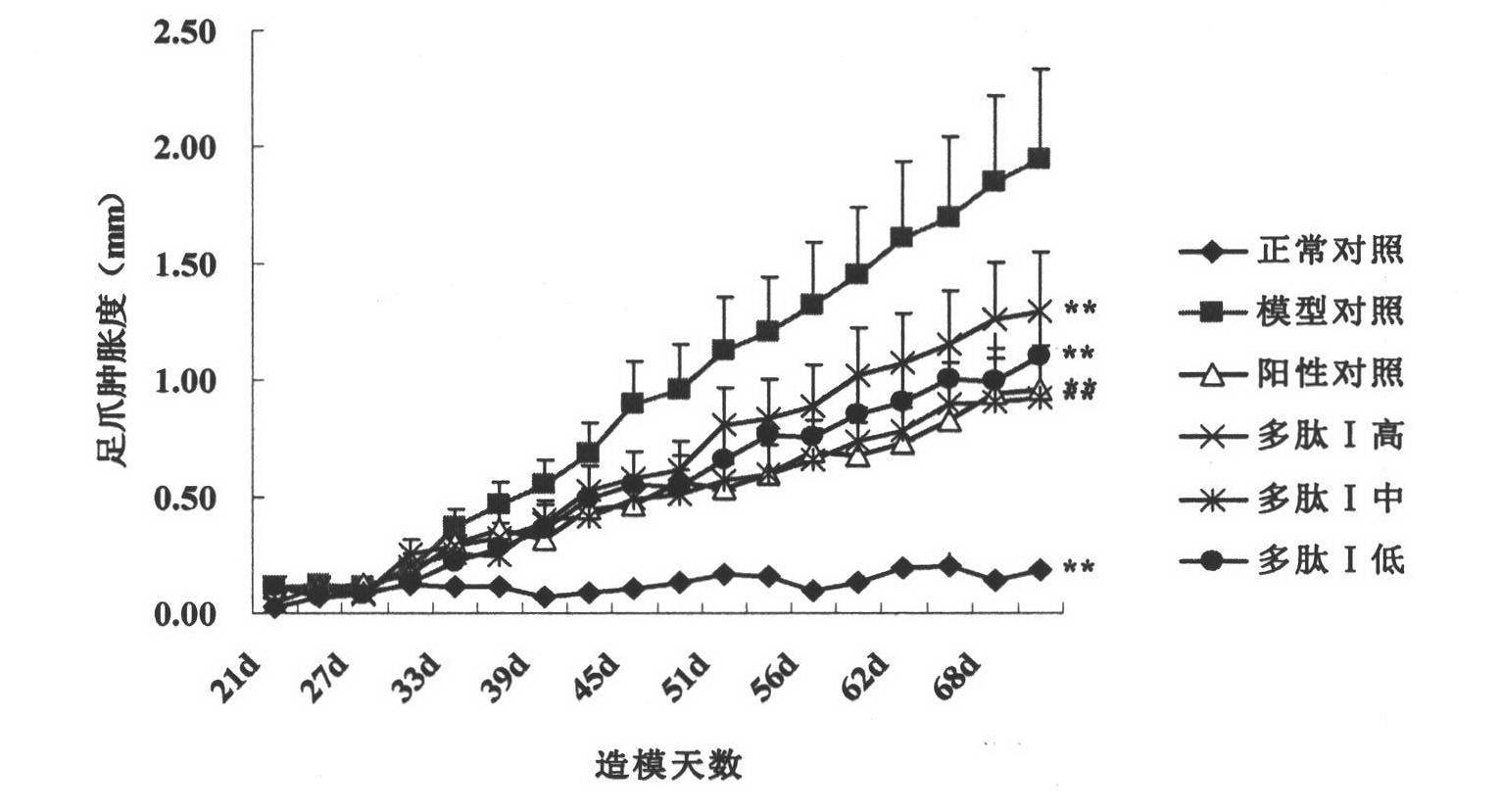
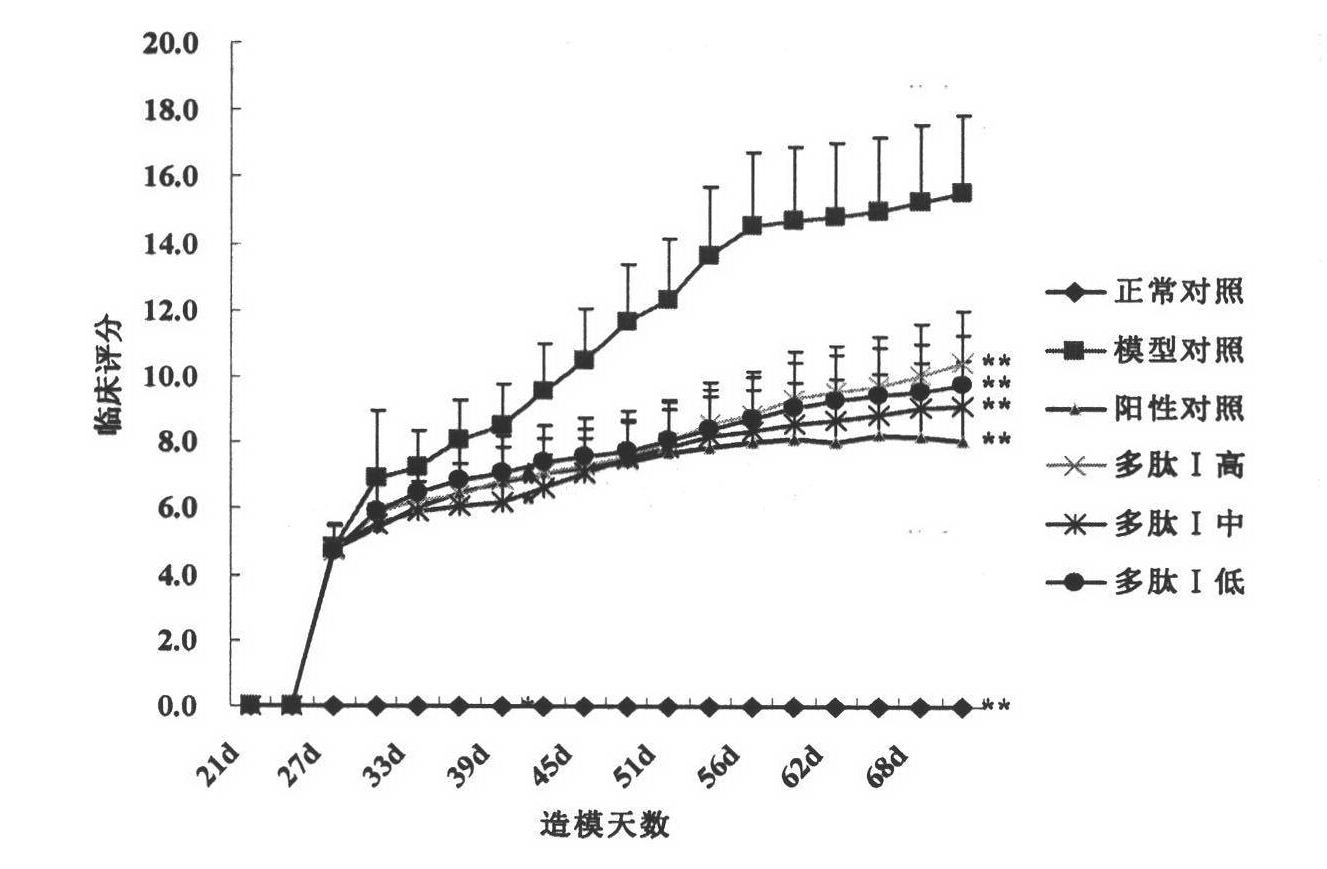
[0050] Immunoprotective Effect of Polypeptide I on Collagen-Induced Mouse Arthritis Animal Model
[0051] A collagen-type mouse arthritis animal model was constructed to study the therapeutic effect of polypeptide on collagen-induced arthritis (CIA) in mice. Mice were used as experimental animals, and 90 SPF grade DBA / 1 mice (provided by Sino-British SIPPR Lab.Animal Ltd), animal production license number: SCXK (Shanghai )2008-0016), male, 7-8 weeks old, with a body weight of 18-22g, were randomly divided into 9 groups, which were normal control group, model control group, 3 low, middle and high dosage groups of polypeptide I (0.2, 0.4, 0.8mg / kg) and positive drug control group (methotrexate 1mg / kg). Except the normal group, mice CIA models were established in each experimental group on day 0 by dissolving chicken cartilage type II collagen (cII) with 0.1 mol / l acetic acid into a 4 mg / ml solution and overnight in a refrigerator at 4°C. On the day of the experiment, fully em...
Embodiment 3
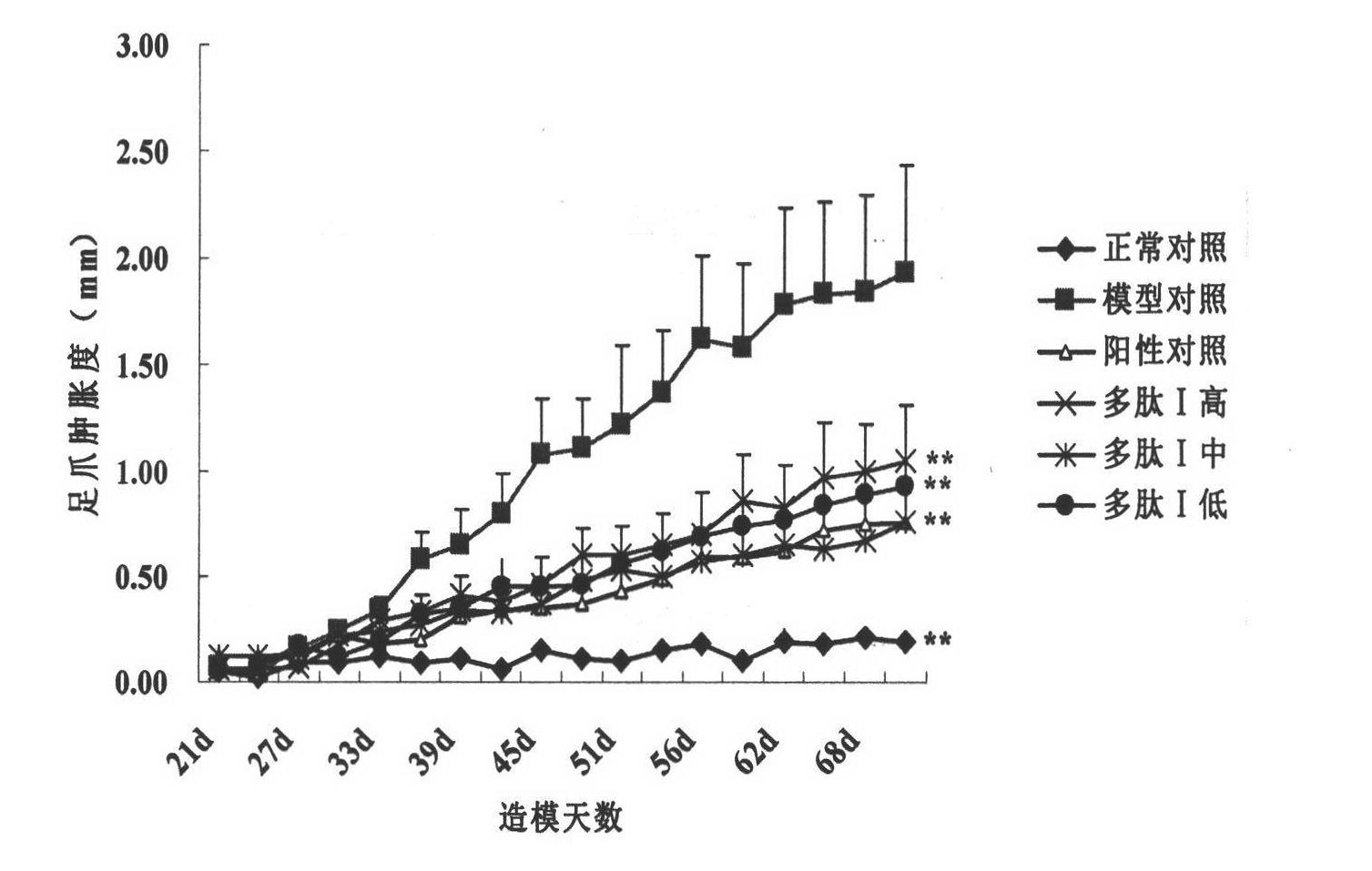
[0066] Protective Effect of Polypeptide I on In Vivo Immunoprotective Effect of Adjuvanted Rat Arthritis Animal Model
[0067] An animal model of adjuvant arthritis in rats was constructed to study the therapeutic effect of the polypeptide on adjuvant arthritis (AA) rats. Rats were used as experimental animals, 90 SPF grade SD rats (provided by Sino-British SIPPR Lab.Animal Ltd), animal production license number: SCXK (Shanghai) 2008 -0016), male, with a body weight of 140g-160g, were randomly divided into 9 groups, which were normal control group, model control group, 3 low, middle and high dose groups of polypeptide I (0.1, 0.2, 0.4mg / kg) and positive drug control group (Methotrexate 1mg / kg). In addition to the normal group, on the 0th day, each experimental group established a rat AA model by injecting complete Freund's adjuvant containing inactivated Mycobacterium tuberculosis (H37RA, 10mg / ml) in the left rear of the rat. 0.08ml caused rat adjuvant arthritis model. Subc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com