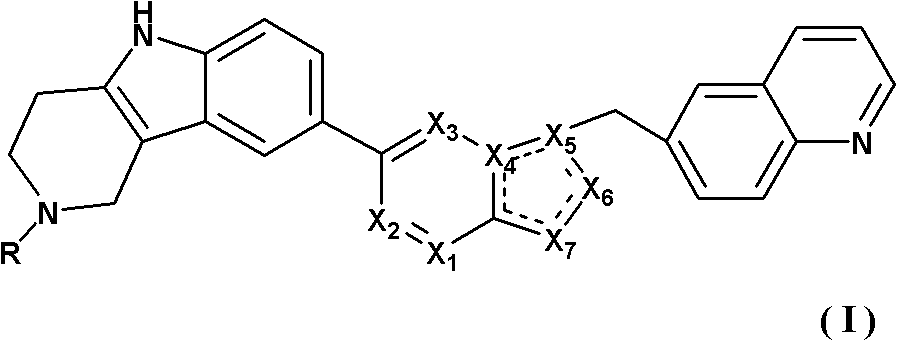
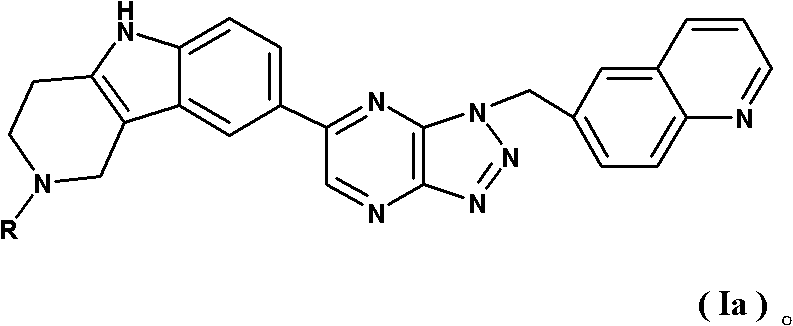
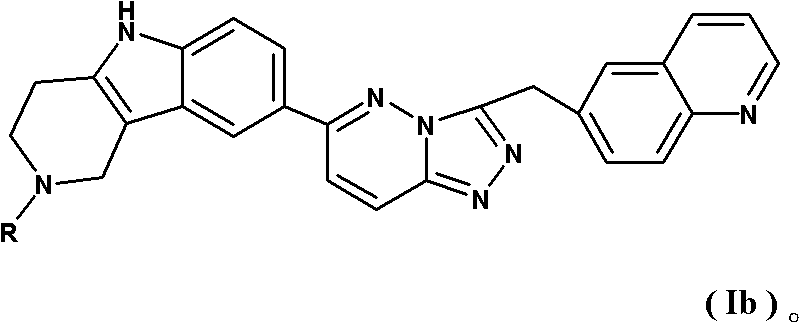
Tricyclic compounds and preparation method thereof, and medicinal composition containing compounds and application thereof
A compound and tricyclic technology, applied in the field of condensed tricyclic compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] 8-[1-(6-quinolinylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]-2,3,4,5-tetra Synthesis of Hydrogen-1H-pyridin[4,3-b]indole
[0191]
[0192] Step 1: Synthesis of 8-bromo-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole
[0193]
[0194] The synthesis method described in this step is slightly improved with reference to the Fischer indole synthesis method (J.-P.Hénichart et al J.Heterocycl.Chem.2006, 43, 571-578), and the specific process is as follows: 4-bromophenylhydrazine Hydrochloride (224 mg or 1 mmol) and N-tert-butoxycarbonyl-4-piperidone (199 mg or 1 mmol) were dissolved in 10 ml of saturated ethanol solution of HCl (prepared by drying HCl gas in absolute ethanol) , the solution was refluxed for 3 hours with stirring, and LC-MS showed that the reaction was complete. After cooling to room temperature, the solvent was removed by rotary evaporation, and the crude product was washed with 10 mL of saturated NaHCO 3 The aqueous solution was worked up and extracted w...
Embodiment 2
[0201] 2-methyl-8-[1-(6-quinolylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]-2,3, Synthesis of 4,5-tetrahydro-1H-pyridin[4,3-b]indole
[0202]
[0203] Step 1: Synthesis of {2-methyl-2,3,4,5-tetrahydro-1H-pyridin[4,3-b]indol-8-yl}boronic acid pinacol ester
[0204]
[0205] {2,3,4,5-tetrahydro-1H-pyridin[4,3-b]indol-8-yl}pinacol borate (1.788 g or 6 mmol) was suspended in 15 mL THF and cooled to -78°C, add dropwise 1M NaN(SiMe 3 ) 2 THF solution (30mL or 30mmol). After the addition was complete, it was stirred at -78°C for 30 minutes, and then 2-chloro-N-(2-chloroethyl)-N-methylethylamine hydrochloride solid (1.155 g or 6 mmol) was added. Stirring was continued for 30 minutes after the addition was complete, then raised to room temperature and stirred for two days. TLC showed that the reaction was complete, and 10 mL of 4M hydrochloric acid aqueous solution was carefully added to the pink suspension, then adjusted to pH ≈ 9 with concentrated ammonia, and extracted ...
Embodiment 3
[0209] 8-[1-(6-quinolinylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]-3,4-dihydro-1H- Synthesis of pyridin[4,3-b]indole-2(5H)-carbaldehyde
[0210]
[0211] Step 1: 8-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydro-1H-pyrido[4, Synthesis of 3-b]indole-2(5H)-carbaldehyde
[0212]
[0213] Formic acid (69 mg or 1.5 mmol) and EDC.HCl (288 mg or 1.5 mmol) were suspended in 20 mL of DCM, and diisopropylethylamine (129 mg or 1 mmol) was added dropwise with stirring. After the addition, stir at room temperature for half an hour, add {2,3,4,5-tetrahydro-1H-pyridin[4,3-b]indol-8-yl}pinacol borate (432mg or 1mmol), Stirring was continued for 2 hours. The solvent was removed by rotary evaporation, and the crude product obtained by concentration was chromatographed on a silica gel column (7M NH 3 methanol solution / DCM: 5 / 95) to obtain 280.4 mg of the target product (yield: 86%). Mass Spectrum m / z: 327.08 [M+H + ].
[0214] Step 2: 8-[1-(6-Quinolinylmethyl)-1H-[1,2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com