Spinal column fusion device internally lined with reinforced ribs
A cage and spine technology, applied in the direction of internal fixator, internal bone synthesis, spinal implants, etc., can solve the problems of reduced intervertebral space and intervertebral foramen height, nerve root damage, and cage loosening, etc. Wide range of options, increased strength, and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
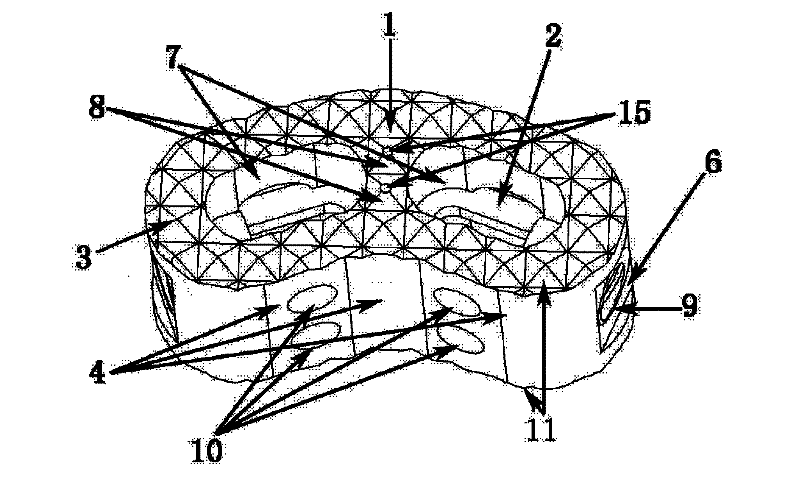
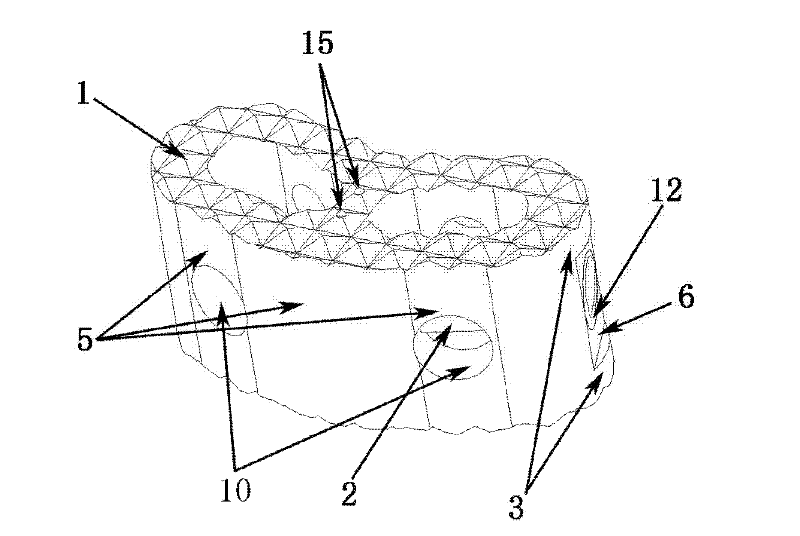
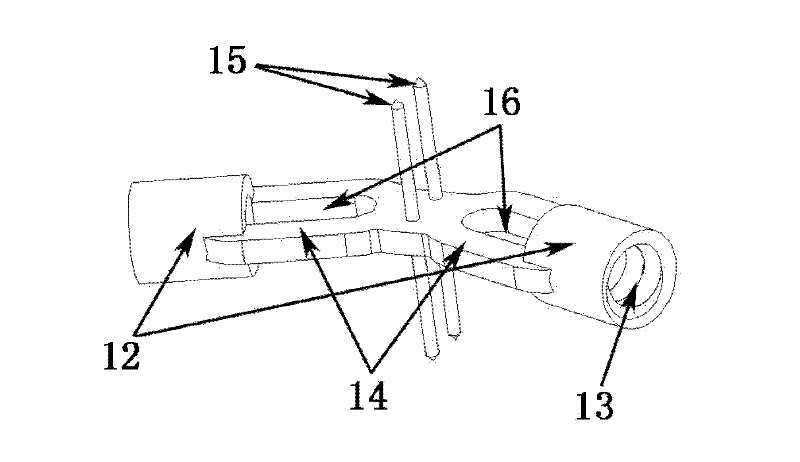
[0039] Such as Figure 1-Figure 6 Shown is the schematic diagram of this embodiment, the main structure 1 of the spinal fusion device is cashew nut-shaped, with an overall width of 14mm, a length of 25mm, a height of 10mm, and a wall thickness of 2.5mm. 0.5mm, can also take any shape that increases the roughness. The base planes of the two bottom surfaces 11 are parallel and have the same shape. The arc-shaped wall in the length direction of the main body is bent to one side at the same time, forming a concave arc-shaped wall 4 on one side and a convex arc-shaped wall 5 on the other side, both of which are left-right symmetrical. The arc-shaped walls on both sides are a plane 6 in the middle section, the main structure includes two hollow holes 7, the two holes have the same shape, and the horizontal direction between the holes is not connected. The cross-sectional shape of the hollow hole 7 is to smoothly connect the arcs at both ends with a straight line. The space occupi...
Embodiment 2
[0043] The structural size of the spinal fusion device is the same as that of Embodiment 1, and the lining reinforcement structure is also the same as that of Embodiment 1. The material used in the spinal fusion cage is the medical implantable PEEK material imported from Germany EVONIK company.
Embodiment 3
[0045] The structural dimensions of the spinal fusion cage are the same as in Embodiment 1, but the fusion cage has an inclination angle of 5 degrees higher at the front and lower at the rear. The width is 14mm, the length is 25mm, the low side is 10mm high, the wall thickness is 2.5mm, and the upper and lower surfaces have anatomical structure. The material used is the medical implantable PEEK material imported by German EVONIK company, which is suitable for patients L4 / L5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com