Perovskite supported nickel base methanation catalyst and preparation method thereof
A methanation catalyst and perovskite technology are applied in the field of perovskite-supported nickel-based synthesis gas complete methanation catalysts and their preparation fields, which can solve the problem of narrow catalyst activity temperature range, catalyst deactivation by carbon deposition, and decreased catalytic efficiency, etc. problem, to achieve the effect of maintaining high activity, good stability, and avoiding the formation of carbonyl groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
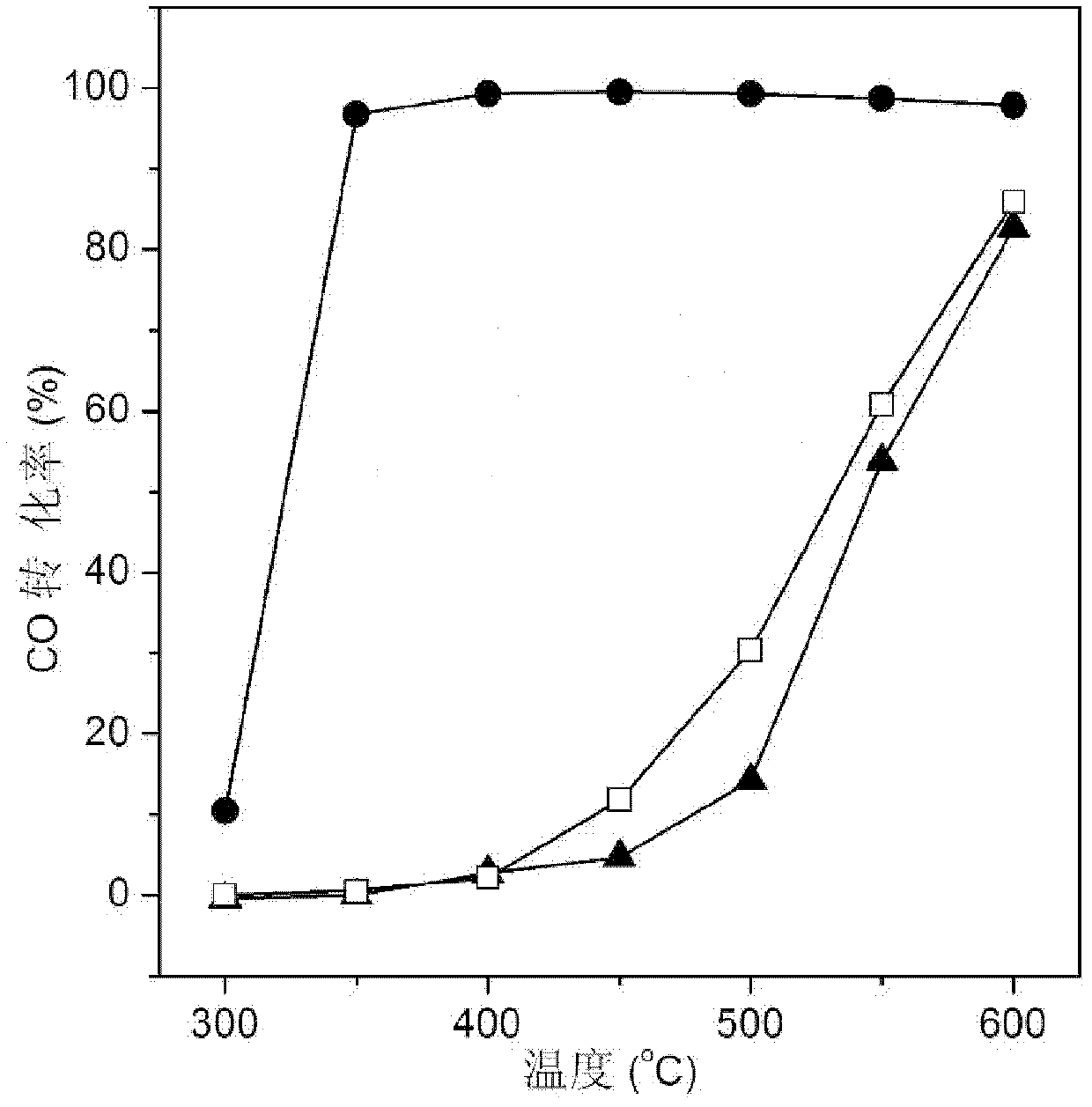
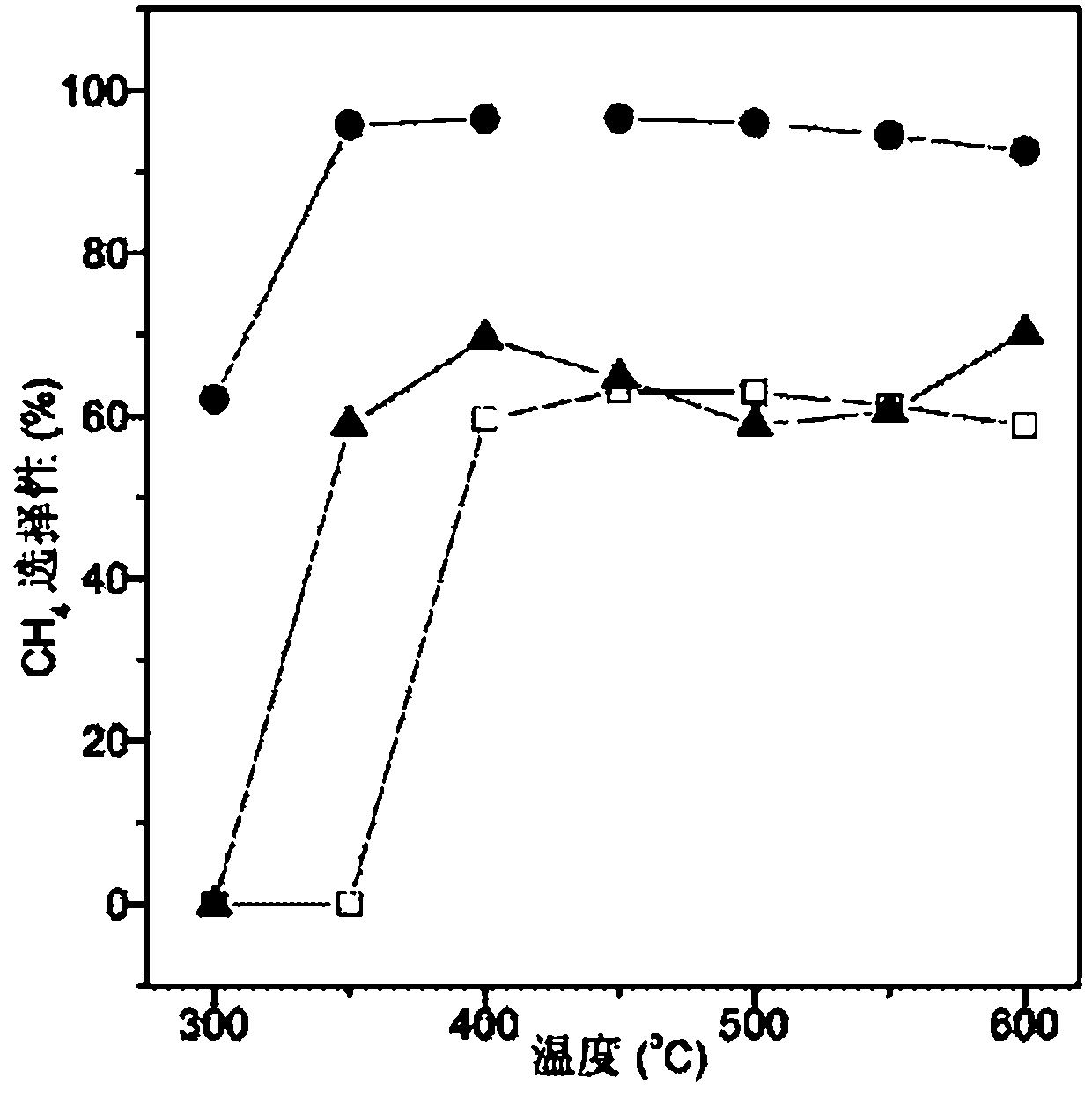
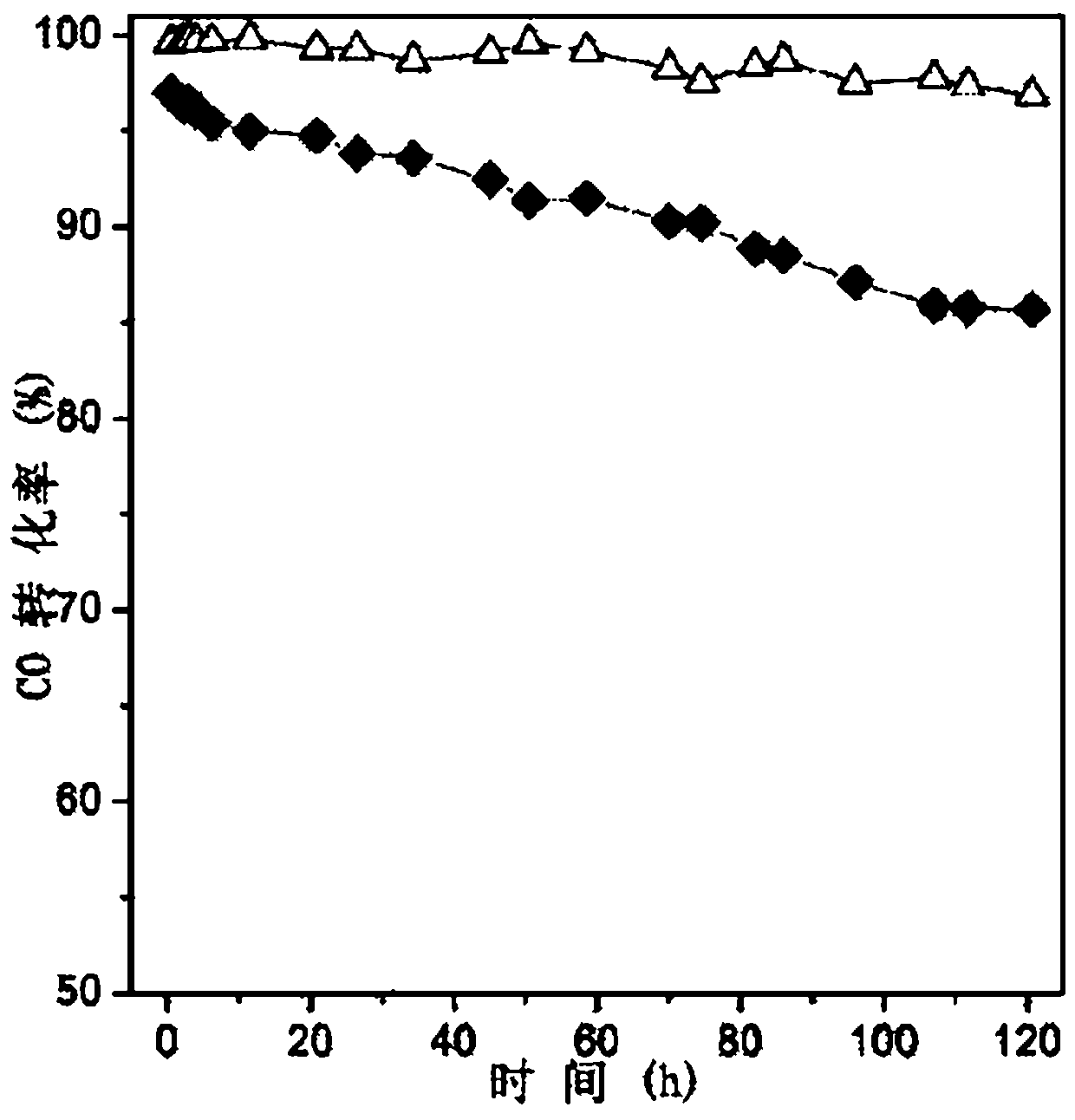
[0054] Commercial CaTiO 3 (specific surface2 / g) placed in a box furnace at 400 ° C for 4 hours, cooled for later use. Weigh 1.8g Ni(AC) 2 4H 2 O, 0.27g La(AC) 3 ·5H 2 O is dissolved in 30g deionized water to form a metal salt solution, and Ni in the solution 2+ The concentration is 0.24mol / L, La 3+ The concentration is 0.022mol / L. Then weigh 10g of calcined CaTiO 3 Add it to the above metal salt solution, stir at 20°C, raise the temperature to 80°C after 2 hours, evaporate to dryness with stirring, put it in an oven and dry it at 120°C for 6 hours, then put it in a box furnace and roast at 400°C 2h, after cooling for later use.
Embodiment 2
[0056] Commercial CaTiO 3 (specific surface2 / g) placed in a box furnace at 400 ° C for 4 hours, cooled for later use. Weigh 4.4g Ni(NO 3 ) 2 ·6H 2 O, 0.61g La(NO 3 ) 3 ·6H 2 O was dissolved in 60g deionized water to form a metal salt solution, in which Ni 2+ The concentration is 0.26mol / L, La 3+ The concentration is 0.024mol / L. Then weigh 10g of calcined CaTiO 3 Add it to the above metal salt solution, stir at 30°C, raise the temperature to 80°C after 8 hours, evaporate to dryness with stirring, put it in an oven and dry it at 100°C for 12 hours, then put it in a box furnace and roast at 400°C for 4 hours , and set aside after cooling.
Embodiment 3
[0058] Commercial CaTiO 3 (specific surface2 / g) placed in a box furnace at 400 ° C for 4 hours, cooled for later use. Weigh 4.4g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 60g deionized water to form a metal salt solution, in which Ni 2+ The concentration is 0.26mol / L. Then weigh 10g of calcined CaTiO 3 Add it to the above metal salt solution, stir at 30°C, raise the temperature to 80°C after 8 hours, evaporate the water with stirring, put it in an oven and dry it at 100°C for 12 hours, then put it in a box furnace and roast at 400°C 4 hours, after cooling for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com