Methyhaaltrexone bromide injection and preparation method thereof
A technology of methylnaltrexone bromide and injection, which is applied in the field of western medicine preparations, can solve problems such as diarrhea, and achieve the effects of preventing deposition, reducing adverse reactions, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
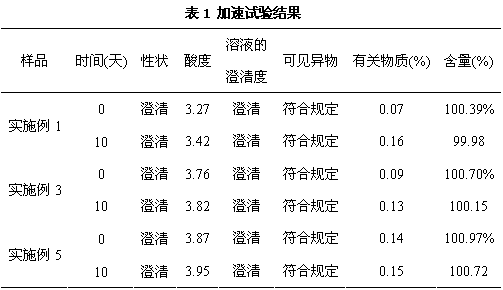
Embodiment 1
[0020] components Dosage Methylnaltrexone bromide 12g Sodium chloride 4g Glycine 0.1g phosphoric acid 0.5g pH 3.0~3.3 Diluted to water for injection 600mL Total 1000 bottles in cans
[0021] Preparation Process:
[0022] (1) Take 80% of the prepared water for injection and put it in the batching tank, add sodium chloride, glycine and phosphoric acid, stir to dissolve and mix evenly, then add bromidenaltrexone, stir to mix evenly;
[0023] (2) Use 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value in an appropriate amount, and add the remainder of water for injection to make up the volume;
[0024] (3) Add 0.1% (g / g) activated carbon for needles, stir at room temperature for 30 minutes, then filter and decarbonize;
[0025] (4) Sterilize and filter the liquid medicine through one 0.45μm and two 0.22μm microporous filter elements respectively;
[0026] (5) Fill the liq...
Embodiment 2
[0028] components Dosage Methylnaltrexone bromide 12g Sodium chloride 4g Glycine 0.1g phosphoric acid 1.5g pH 3.3~3.5 Diluted to water for injection 600mL Total 1000 bottles in cans
[0029] Preparation Process:
[0030] (1) Take 80% of the prepared water for injection and put it in the batching tank, add sodium chloride, glycine and phosphoric acid, stir to dissolve and mix evenly, then add bromidenaltrexone, stir to mix evenly;
[0031] (2) Use 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value in an appropriate amount, and add the remainder of water for injection to make up the volume;
[0032] (3) Add 0.1% (g / g) activated carbon for needles, stir at room temperature for 30 minutes, then filter and decarbonize;
[0033] (4) Sterilize and filter the liquid medicine through one 0.45μm and two 0.22μm microporous filter elements respectively;
[0034] (5) Fill the liq...
Embodiment 3
[0036] components Dosage Methylnaltrexone bromide 12g Sodium chloride 3.9g Glycine 0.2g phosphoric acid 1g pH 3.5~4.0 Diluted to water for injection 600mL Total 1000 bottles in cans
[0037] Preparation Process:
[0038] (1) Take 80% of the prepared water for injection and put it in the batching tank, add sodium chloride, glycine and phosphoric acid, stir to dissolve and mix evenly, then add methylnaltrexone bromide, stir to mix evenly;
[0039] (2) Use 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution to adjust the pH value in an appropriate amount, and add the remainder of water for injection to make up the volume;
[0040] (3) Add 0.1% (g / g) activated carbon for needles, stir at room temperature for 30 minutes, then filter and decarbonize;
[0041] (4) Sterilize and filter the liquid medicine through one 0.45μm and two 0.22μm microporous filter elements respectively;
[0042] (5) Fill ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com