Octreotide acetate freeze-dried combination for injection and preparation method thereof
A technology for octreotide acetate and a composition, which is applied in the field of pharmacy, can solve the problems of microbial contamination, hidden dangers, long half-life, etc., and achieves the effects of improving stability, overcoming local irritation, good shelf life stability and compatibility stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
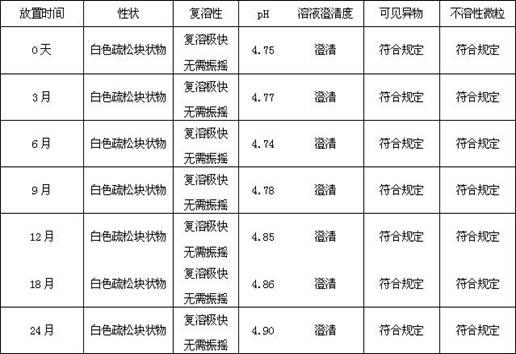
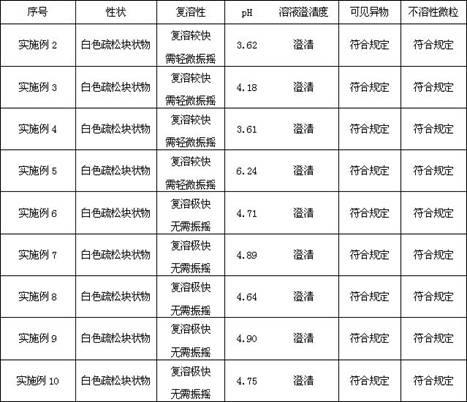
Examples
Embodiment 1
[0032] Embodiment 1 Pre-experiment
[0033] 1. During the preparation of octreotide acetate solution, the optimal pH of the solution was determined by examining the stability of solutions with different pH values, and at the same time, different pH buffer pairs were investigated to examine whether they were suitable for the prescription.
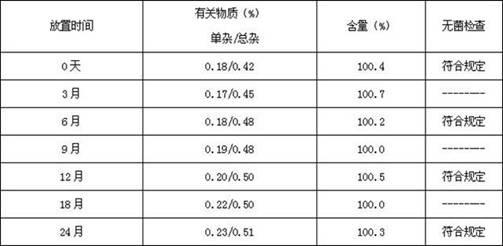
[0034] Table 1 Stability investigation of octreotide acetate solutions with different pHs (the index is the content of related substances, 20°C)
[0035] pH 0h 1h 2h 4h 8h 3.5 0.52% 0.60% 0.67% 0.78% 0.90% 4.0 0.48% 0.57% 0.64% 0.74% 0.88% 4.5 0.40% 0.42% 0.42% 0.44% 0.49% 5.0 0.47% 0.40% 0.43% 0.45% 0.48% 5.5 0.55% 0.62% 0.73% 0.80% 0.94% 6.0 0.58% 0.69% 0.80% 0.94% 1.15%
[0036] The study found that the octreotide acetate solution had better stability in the pH range of 4.5 to 5.0. In the investigation of different buffer systems, we found that lactic acid / sodium bic...
Embodiment 2
[0050] The pH of the solution does not fall into the optimal pH range, the temperature of the solution preparation is not controlled at a low level, and the freezing and pre-freezing method is medium freezing
[0051] prescription
[0052] Octreotide Acetate
[0053] (C 49 h 66 N 10 o 10 S 2 ) 0.1g
[0054] Mannitol 45g
[0055] Lactic acid 3.4g
[0056] Sodium bicarbonate to adjust pH to 3.5
[0057] Add water for injection to 1000ml
[0058]
[0059] Makes 1000 bottles
[0060] Take the prescribed amount of mannitol, lactic acid and water for injection about 800ml, stir to dissolve, add 0.1% activated carbon, heat to 80°C and stir for 30 minutes, decarbonize through titanium rod circulation, cool the filtrate to 30°C, add the prescribed amount of octreotide acetate After stirring and dissolving, add sodium bicarbonate to adjust the pH to 3.5, and add water for injection to 1000ml. Send it to a sterile room by a peristaltic pump, ...
Embodiment 3
[0064] The pH of the solution does not fall within the optimal pH range, the solution preparation temperature is not controlled at a low level, and the freezing and pre-freezing are prescribed in the middle freezing method
[0065] Octreotide Acetate
[0066] (C 49 h 66 N 10 o 10 S 2 ) 0.1g
[0067] Mannitol 45g
[0068] Tartaric acid 3.0g
[0069] Sodium tartrate Adjust pH to 4.0
[0070] Add water for injection to 1000ml
[0071]
[0072] Makes 1000 bottles
[0073] Take the prescribed amount of mannitol, tartaric acid and water for injection about 800ml, stir to dissolve, add 0.1% activated carbon, heat to 80°C and stir for 30 minutes, decarbonize through titanium rod circulation, cool the filtrate to 30°C, add the prescribed amount of octreotide acetate After stirring and dissolving, add sodium tartrate to adjust the pH to 4.0, and add water for injection to 1000ml. Send it to a sterile room by a peristaltic pump, filter it thro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com