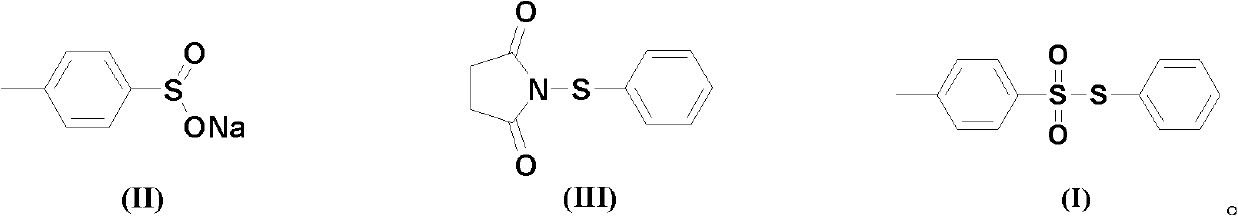
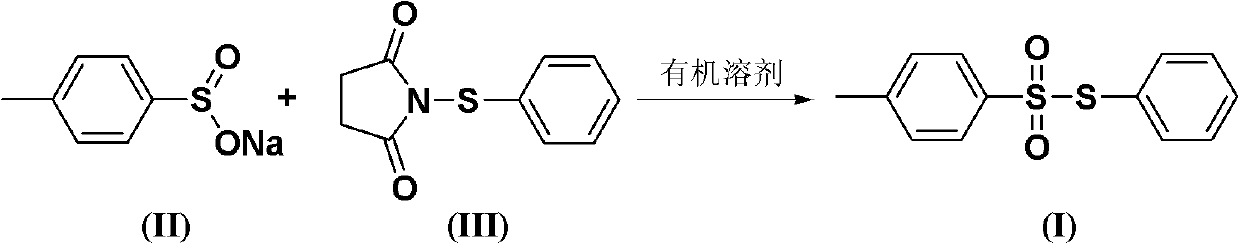
Chemical synthesis method of S-phenyl-4-tosylate
A technology of tosylate and chemical synthesis, applied in organic chemistry and other directions, can solve the problems of high risk factor, poor stability, high volatility, etc., and achieve the effects of environmental friendliness, low production cost, and three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The ratio of N-phenylmercaptosuccinimide and sodium p-toluenesulfinate is 1:1.0 according to the amount of feed material, N-phenylmercaptosuccinimide 20.7g (0.1mol); sodium p-toluenesulfinate 17.8g (0.1mol); The organic solvent is toluene 207g, and its total consumption is 10 times of the quality of N-phenylmercaptosuccinimide.
[0019] Sodium p-toluene sulfinate is dissolved in an organic solvent (the amount of the organic solvent is 6 times the mass of N-phenylmercaptosuccinimide). N-phenylmercaptosuccinimide is dissolved in an organic solvent (organic solvent consumption is 4 times of the quality of N-phenylmercaptosuccinimide), slowly added dropwise to the solution of sodium p-toluenesulfinate, the reaction temperature The temperature was 50°C, and the reaction was completed after 6 hours.
[0020] After the reaction was completed, the resulting reaction solution was added with saturated brine, extracted and separated to take the organic layer, and the organic laye...
Embodiment 2
[0023] The ratio of N-phenylmercaptosuccinimide and sodium p-toluenesulfinate is 1:1.5 according to the amount of feed material, N-phenylmercaptosuccinimide 20.7g (0.1mol); sodium p-toluenesulfinate 26.7g (0.15mol); The organic solvent is toluene 207g, and its total consumption is 10 times of the quality of N-phenylmercaptosuccinimide.
[0024] Other operations were the same as in Example 1 to obtain 23.2 g of S-phenyl-4-toluenesulfonate, with a yield of 88% and a purity of 98.8%.
Embodiment 3
[0026] The ratio of N-phenylmercaptosuccinimide and sodium p-toluenesulfinate is 1:1.5 according to the amount of feed material, N-phenylmercaptosuccinimide 20.7g (0.1mol); sodium p-toluenesulfinate 26.7g (0.15mol); the organic solvent is 207g of n-hexane, and its total consumption is 10 times of the quality of N-phenylmercaptosuccinimide.
[0027] The reaction temperature was 30°C and the reaction time was 7 hours. Other operations were the same as in Example 1 to obtain 21.9 g of S-phenyl-4-toluenesulfonate with a yield of 83% and a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com