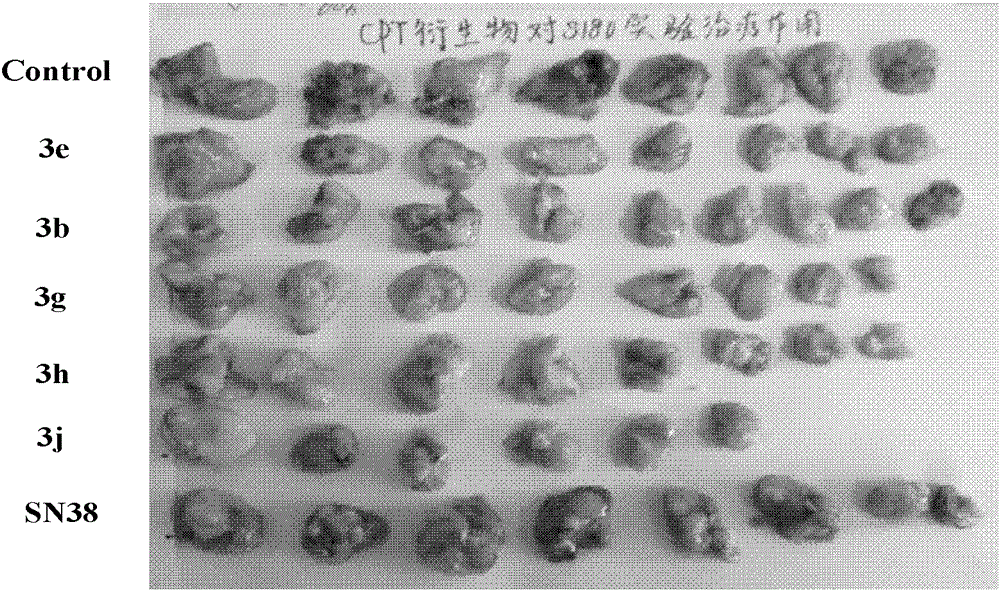
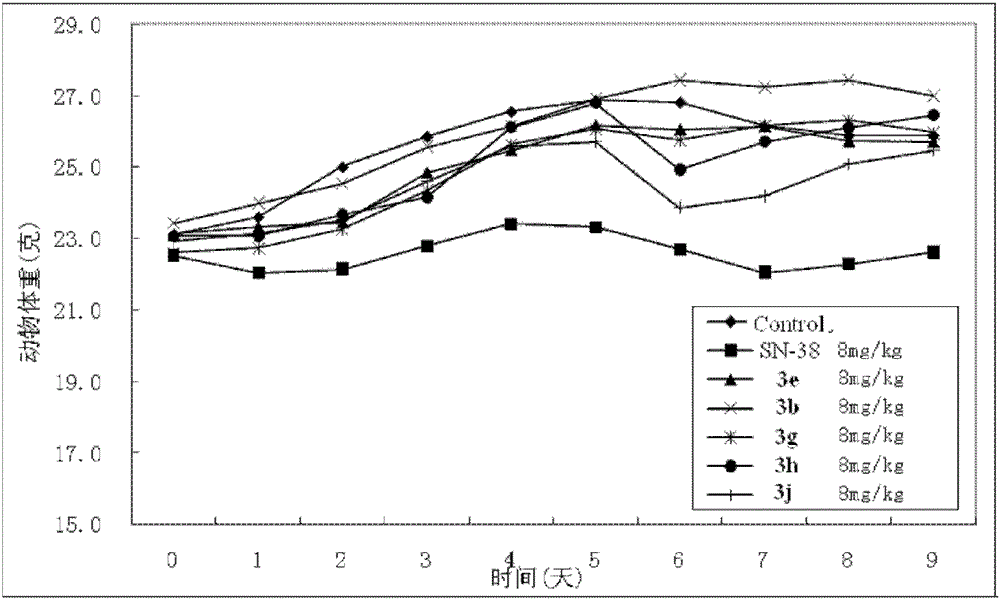
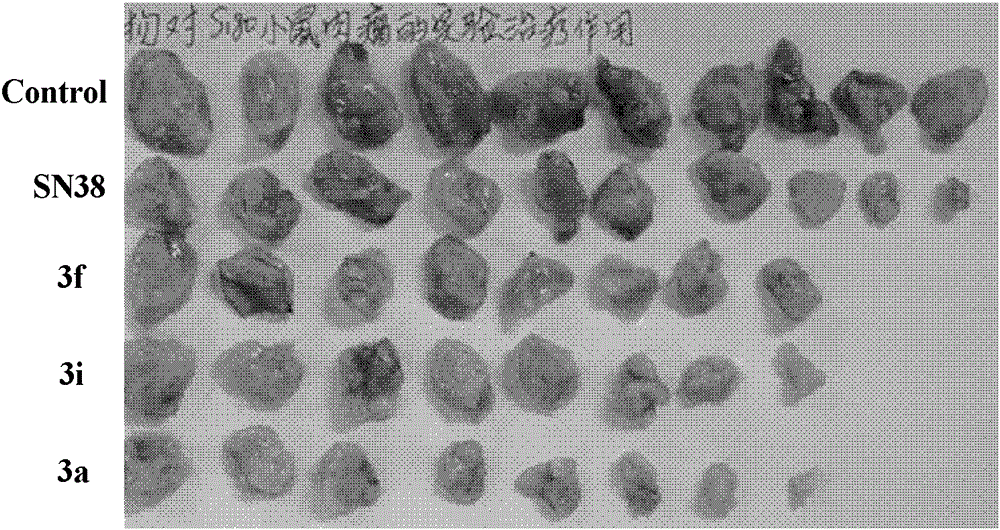
7-Ethyl-20(s)-o-substituted benzoylcamptothecin compounds with antitumor activity
A technology for nitrobenzoyl camptothecin and benzoyl camptothecin, which is applied in the field of pharmaceutical compounds, can solve the problems of stagnation of clinical research, poor water solubility of natural camptothecin, and achieves low growth and metabolism and no weight gain. Inhibitory action, effect of low toxicity properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] Instruments and reagents used in the following examples: X-4 type digital display micro melting point instrument (the thermometer is not calibrated); EssentiaLC-15C type high performance liquid chromatography (Luna octadecyl silica gel column, mobile phase is methanol- Water 80:20, 267nm); BRUKERAVANCE AV-500 nuclear magnetic resonance spectrometer (CDCl 3 as solvent, TMS as internal standard); Thermo Nicolet iS10 infrared spectrometer (KBr pellet); Waters Q-TOF MicroTM mass spectrometer.
[0066] Camptothecin was provided by Chengdu Lanqi Pharmaceutical Co., Ltd., EDCI and DMAP were provided by Suzhou Haofan Biotechnology Co., Ltd. Other raw materials and reagents used were analytically pure, CH 2 Cl 2 CaH 2 Reflux drying treatment, n-propanal redistilled before use.
[0067] Synthesis of 7-ethylcamptothecin (2):
[0068] Dissolve 3.00 g (8.60 mmol) of camptothecin (1) in 80 mL of glacial acetic acid, slowly add 20 mL of 98% sulfuric acid dropwise at 5-10°C, and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com