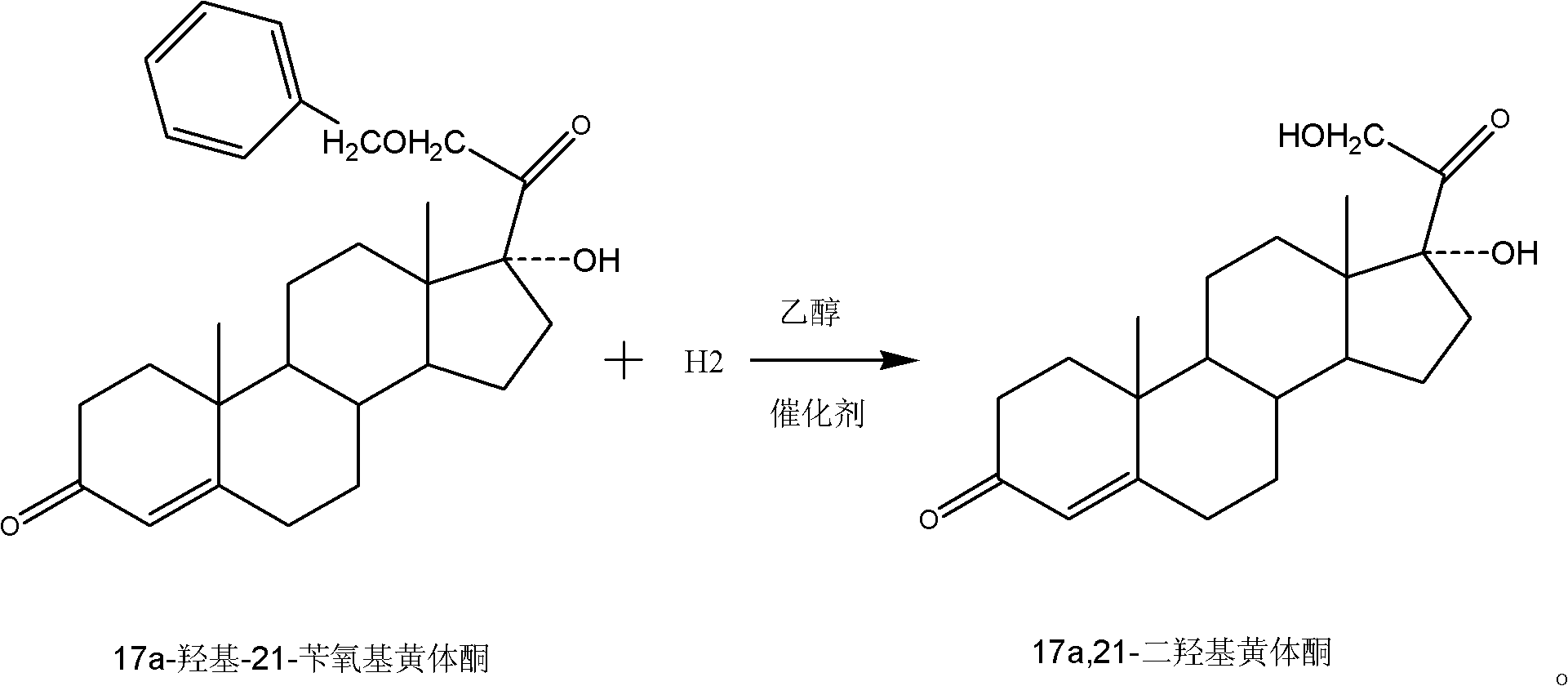
Preparation method for 17a,21-dyhydroxy progesterone
A technology of progesterone and dihydroxy, which is applied in the field of organic compound preparation, can solve the problems of complex preparation process, poor reproducibility and high cost, and achieve the effect of high yield, low cost and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
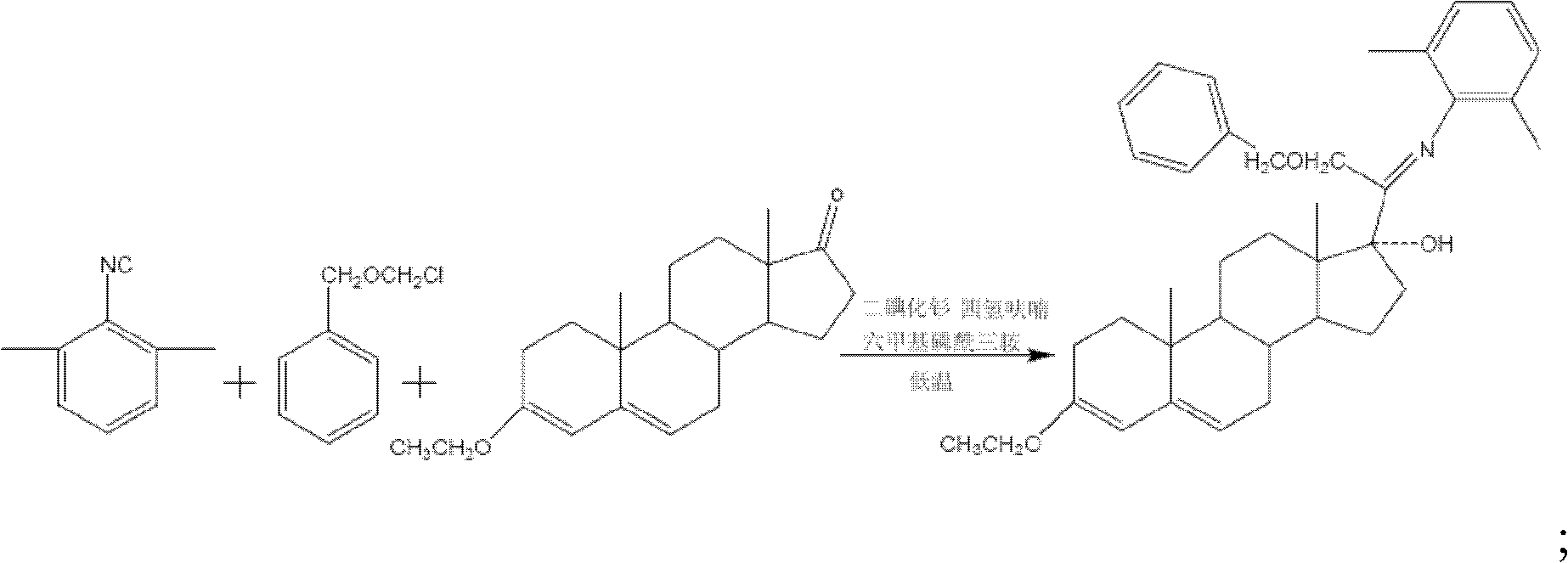
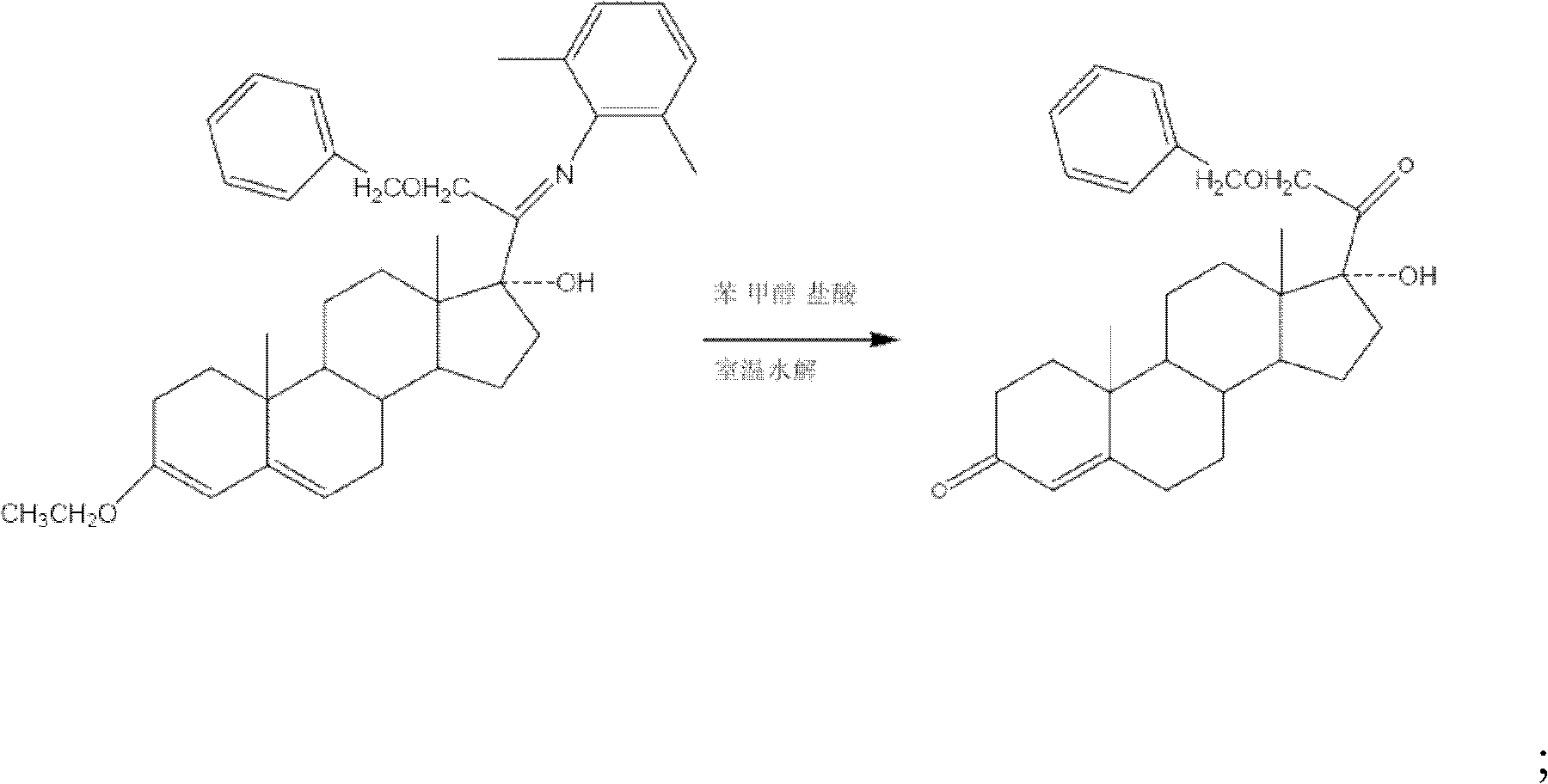
[0028] (1) Preparation of 3-ethoxy-17a-hydroxy-20-(arimino)-21-benzyloxyprogesterone.
[0029] First prepare 3-ethoxyandrost-3,5-dien-17-one:
[0030] Add 10g of androst-4-ene-3,17-dione and 10ml of absolute ethanol into the reaction flask, add 0.1g of p-toluenesulfonic acid and 10ml of triethyl orthoformate under stirring, heat up to 40°C and keep for 3 hours to get. Melting point 145 to 146°C.
[0031] Then prepare 3-ethoxyl-17a-hydroxyl-20-(arimino)-21-benzyloxyprogesterone: under the protection of nitrogen gas into the reactor, the tetrahydrofuran solution of 0.1mol / L samarium diiodide 15L was added to the reactor, under stirring, 56g of 2,6-dimethylbenzene isonitrile and 700ml of hexamethylphosphoric triamide were added, cooled to -15°C with cold brine, and 117.5g of benzyloxymethyl chloride was added dropwise After the addition, stir at this temperature for 3 hours, then add 78.5g of 3-ethoxyandrost-3,5-dien-17-one, react at 0°C to 3°C for 14 hours, and confirm the re...
Embodiment 2
[0041] This example uses the 3-ethoxyandrost-3,5-dien-17-one prepared in Example 1.
[0042](1) Preparation of 3-ethoxyl-17a-hydroxyl-20-(arimino)-21-benzyloxyprogesterone: under the protection of nitrogen gas into the reactor, 0.1mol / L samarium diiodide Add 15L of tetrahydrofuran solution into the reactor, under stirring, add 50g of 2,6-dimethylbenzene isonitrile and 700ml of hexamethylphosphoric triamide, cool to -15°C with cold brine, add benzyloxymethyl chloride dropwise 110g, after adding, stir at this temperature for 3 hours, then add 70g of 3-ethoxyandrost-3,5-dien-17-one, react at 0℃~3℃ for 14 hours, confirm the reaction by thin layer chromatography End point (composition of developing agent: n-hexane:ethyl acetate=5:5), when the raw material spots disappear, the reaction ends. Then add a small amount of water and 3L of n-hexane to stir and filter, the filtrate is allowed to stand for stratification, and the organic layer is distilled off the solvent under reduced pre...
Embodiment 3
[0052] This example uses the 3-ethoxyandrost-3,5-dien-17-one prepared in Example 1.
[0053] (1) Preparation of 3-ethoxyl-17a-hydroxyl-20-(arimino)-21-benzyloxyprogesterone: under the protection of nitrogen gas into the reactor, 0.1mol / L samarium diiodide Add 15L of tetrahydrofuran solution into the reactor, under stirring, add 60g of 2,6-dimethylbenzene isonitrile and 700ml of hexamethylphosphoric triamide, cool to -15°C with cold brine, add benzyloxymethyl chloride dropwise 130g, after adding, stir at this temperature for 3 hours, then add 80g of 3-ethoxyandrost-3,5-dien-17-one, react at 0℃~3℃ for 14 hours, confirm the reaction by thin layer chromatography End point (composition of developing agent: n-hexane:ethyl acetate=5:5), when the raw material spots disappear, the reaction ends. Then add a small amount of water and 3L of n-hexane to stir and filter, the filtrate is allowed to stand for stratification, and the organic layer is distilled off the solvent under reduced pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com