Reduction preparation method of 4-(trifluoromethyl)piperidine
A technology for trifluoromethylpiperidine and trifluoromethylpyridine is applied in the field of reduction preparation for preparing 4-trifluoromethylpiperidine and 4-trifluoromethylpiperidine, and can solve the problems of expensive raw materials, complicated processes, problems such as low output, to achieve the effects of convenient preparation, simple process, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A reduction preparation method of 4-trifluoromethylpiperidine, comprising the following steps:
[0023] (1) Preparation of 4-trifluoromethylpiperidine:
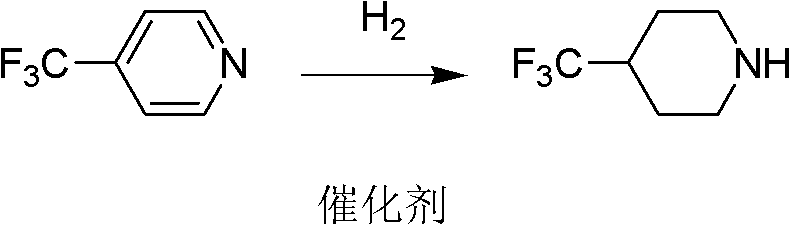
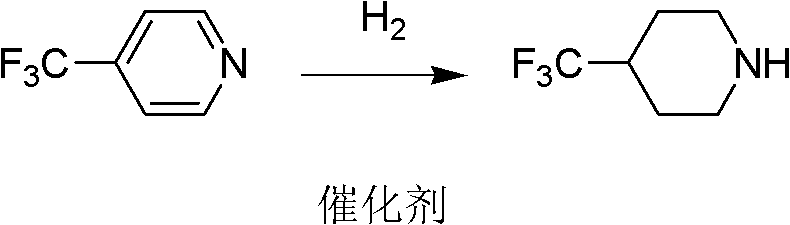
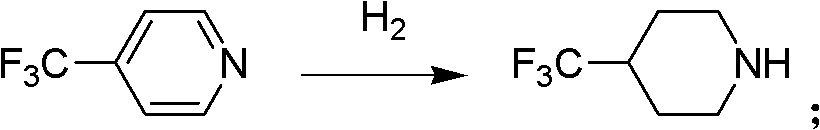
[0024] At room temperature, 4-trifluoromethylpyridine and palladium-carbon are put into the reactor, and the amount of palladium-carbon catalyst is 2% of the 4-trifluoromethylpyridine weight, and hydrogen is passed into the reactor and kept for 3 ~12 atmospheric pressure, carry out hydrogenation under stirring, carry out hydrogenation reduction reaction under the catalysis of palladium carbon, generate 4-trifluoromethylpiperidine, reaction is shown in formula 2:
[0025]
[0026]
[0027] Formula 2
[0028] (2) Purification of 4-trifluoromethylpiperidine:
[0029] The reaction mixture in the step (1) is filtered to remove the catalyst, and the obtained filtrate is concentrated by distillation to remove ethanol. The temperature of the distillation concentration is 100° C., distillation, and the distillation conc...
Embodiment 2
[0032] A reduction preparation method of 4-trifluoromethylpiperidine, comprising the following steps:
[0033] (1) Preparation of 4-trifluoromethylpiperidine:
[0034] At room temperature, 4-trifluoromethylpyridine and Raney nickel are put into the reactor, the addition of Raney nickel catalyst is 8% of 4-trifluoromethylpyridine weight, feed hydrogen into the reactor and Maintain 3-12 atmospheric pressure, carry out hydrogenation under stirring, and carry out hydrogenation reduction reaction under the catalysis of Raney nickel to generate 4-trifluoromethylpiperidine, and the reaction is shown in formula 2.
[0035] (2) Purification of 4-trifluoromethylpiperidine:
[0036] The reaction mixture in step (1) was filtered to remove the catalyst, and the obtained filtrate was concentrated by distillation to remove ether. The temperature of the distillation concentration was 200 ° C. Distillation. The distillation concentration step used a rotary evaporator, and the concentrate was ...
Embodiment 3
[0039] A reduction preparation method of 4-trifluoromethylpiperidine, comprising the following steps:
[0040] (1) Preparation of 4-trifluoromethylpiperidine:
[0041] At room temperature, 4-trifluoromethylpyridine and Raney nickel are put into the reactor, the addition of Raney nickel catalyst is 15% of 4-trifluoromethylpyridine weight, feed hydrogen into the reactor and Maintain 3-12 atmospheric pressure, carry out hydrogenation under stirring, and carry out hydrogenation reduction reaction under the catalysis of Raney nickel to generate 4-trifluoromethylpiperidine, and the reaction is shown in formula 2.
[0042] (2) Purification of 4-trifluoromethylpiperidine:
[0043] The reaction mixture in the step (1) is filtered to remove the catalyst, and the obtained filtrate is concentrated by distillation to remove propylene glycol. The temperature of the distillation concentration is 150°C. Distillation. The distillation concentration step uses a rotary evaporator, and the conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com