Biological and haemostatic wound dressing and preparation method thereof
A hemostatic dressing and biological technology, applied in the fields of medical science, absorbent pads, bandages, etc., can solve the problems of not meeting clinical requirements, unsatisfactory retention time, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of biological hemostatic dressing
[0045] The biological hemostatic dressing is prepared by the method of the present invention, comprising the following steps:
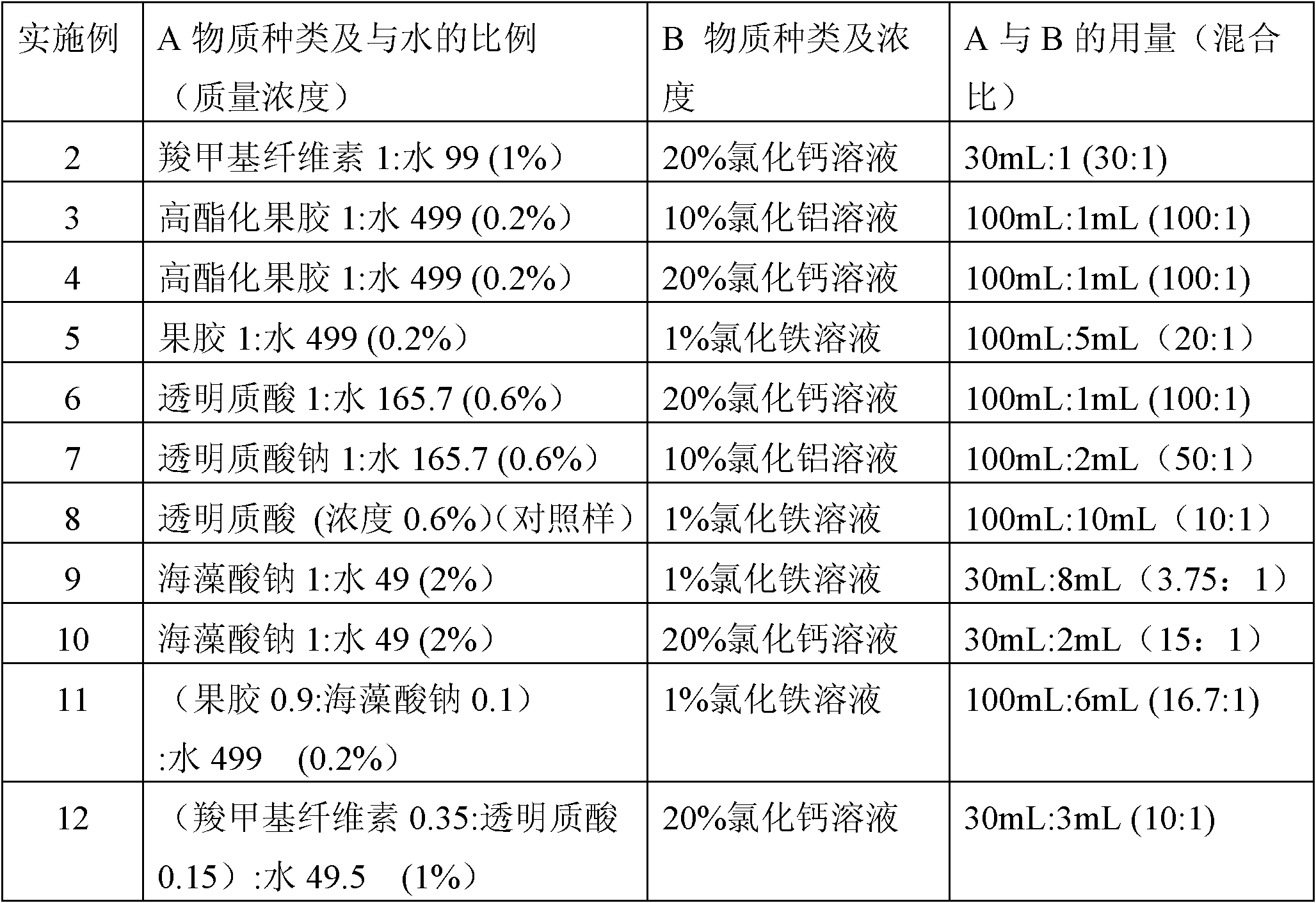
[0046] 1) Preparation of cross-linked gel: 1 g of a specific substance A, carboxymethyl cellulose, was added to 99 ml (g) of water (1:99) to obtain an aqueous solution of carboxymethyl cellulose with a mass concentration of 1%. 3 hours to dissolve or swell, adjust the aqueous solution to pH 4-6 with 0.1N HCl; then mix 30 mL of carboxymethyl cellulose aqueous solution with 1 mL of a specific B substance mass concentration of 10% aluminum chloride solution (mixing ratio 30:1), after mixing, place at room temperature for 2 hours until it is cross-linked into a water-insoluble gel; neutralize the gel with 0.2N aqueous sodium hydroxide solution to neutral pH, and wash with distilled water 3 times, funnel filtration, and the cross-linked gel was obtained after removing the filtrate. ...
Embodiment 2-10
[0049] Embodiment 2-10, the preparation of biological hemostatic dressing
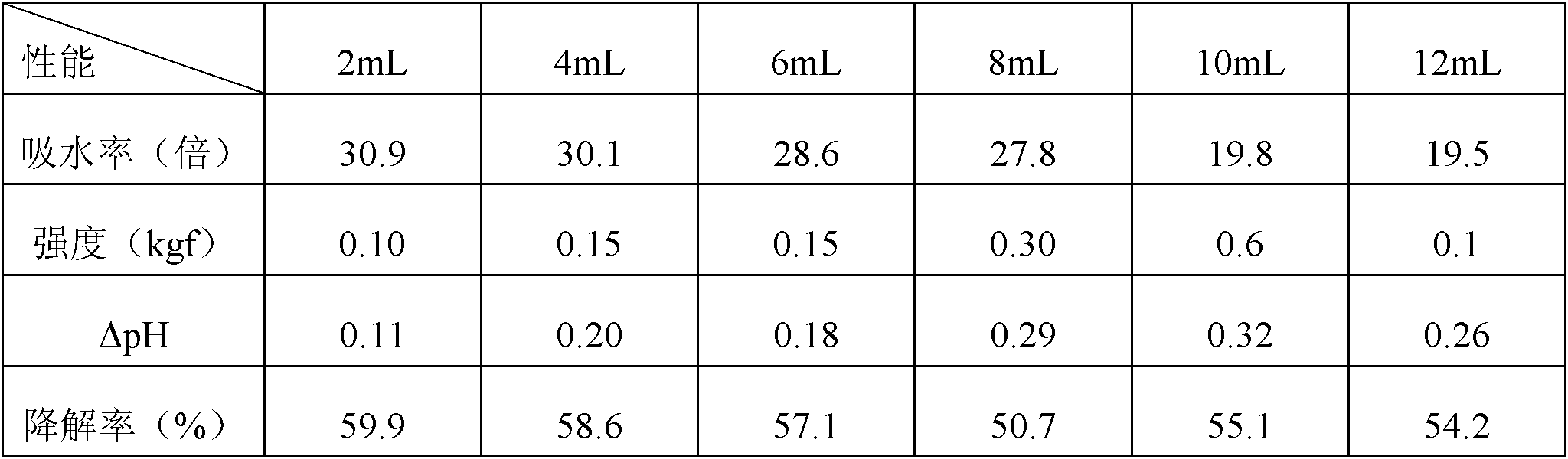
[0050] Different biological hemostatic dressings were prepared by the same method as in Example, with the changes listed in Table 1:
[0051] Table 1: Optimized ratio of biological hemostatic dressings
[0052]
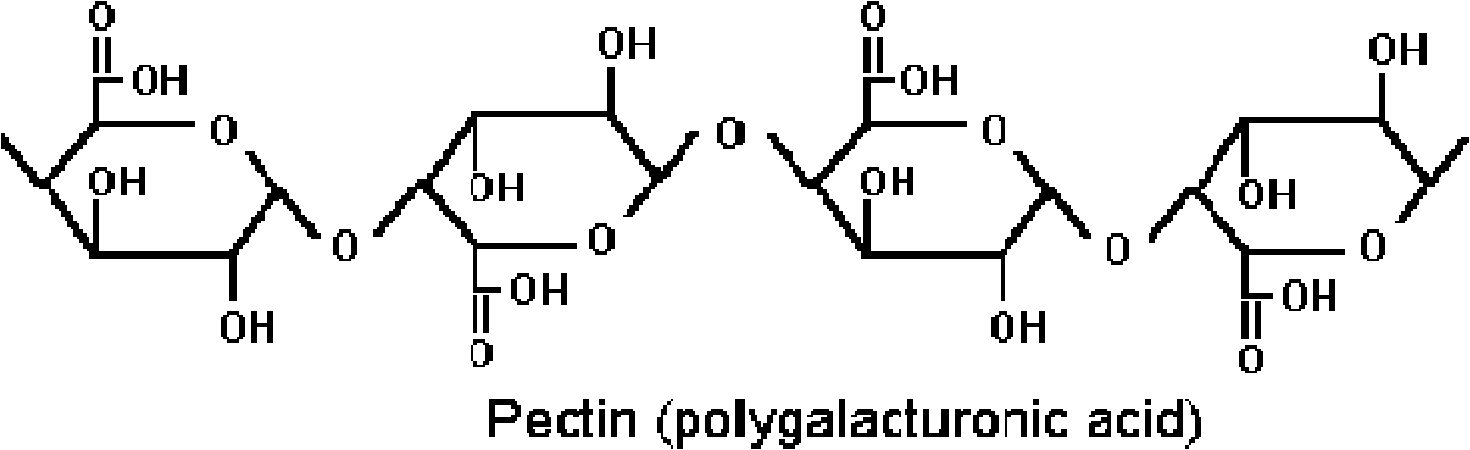
[0053] Note: Instructions for high esterified pectin:
[0054] Pectin with an esterification degree greater than 50% is high-esterification pectin; pectin with an esterification degree less than 50% is low-esterification pectin. High-esterified pectin means that more than half of the carboxyl groups in high-methoxy pectin exist in the form of methyl esterification (-COOCH3), and the remaining carboxyl groups exist in the form of free acid (-COOH) and salt (-COONa).
[0055] The structure of pectin is
[0056]
[0057] Manufacturers: Guangzhou Jianke Biotechnology Co., Ltd., Guangdong Xinyuehai Commercial Development Co., Ltd., Quzhou Pectin Co., Ltd., Guangzhou Yexing Trading Co., Ltd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com