Method for preparing epsilon-caprolactone
A technology of caprolactone and cyclohexanone, which is applied in the field of producing ε-caprolactone, can solve problems such as the need to determine process conditions, achieve stable reaction temperature control, reduce the possibility of safety problems, and improve heat exchange capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
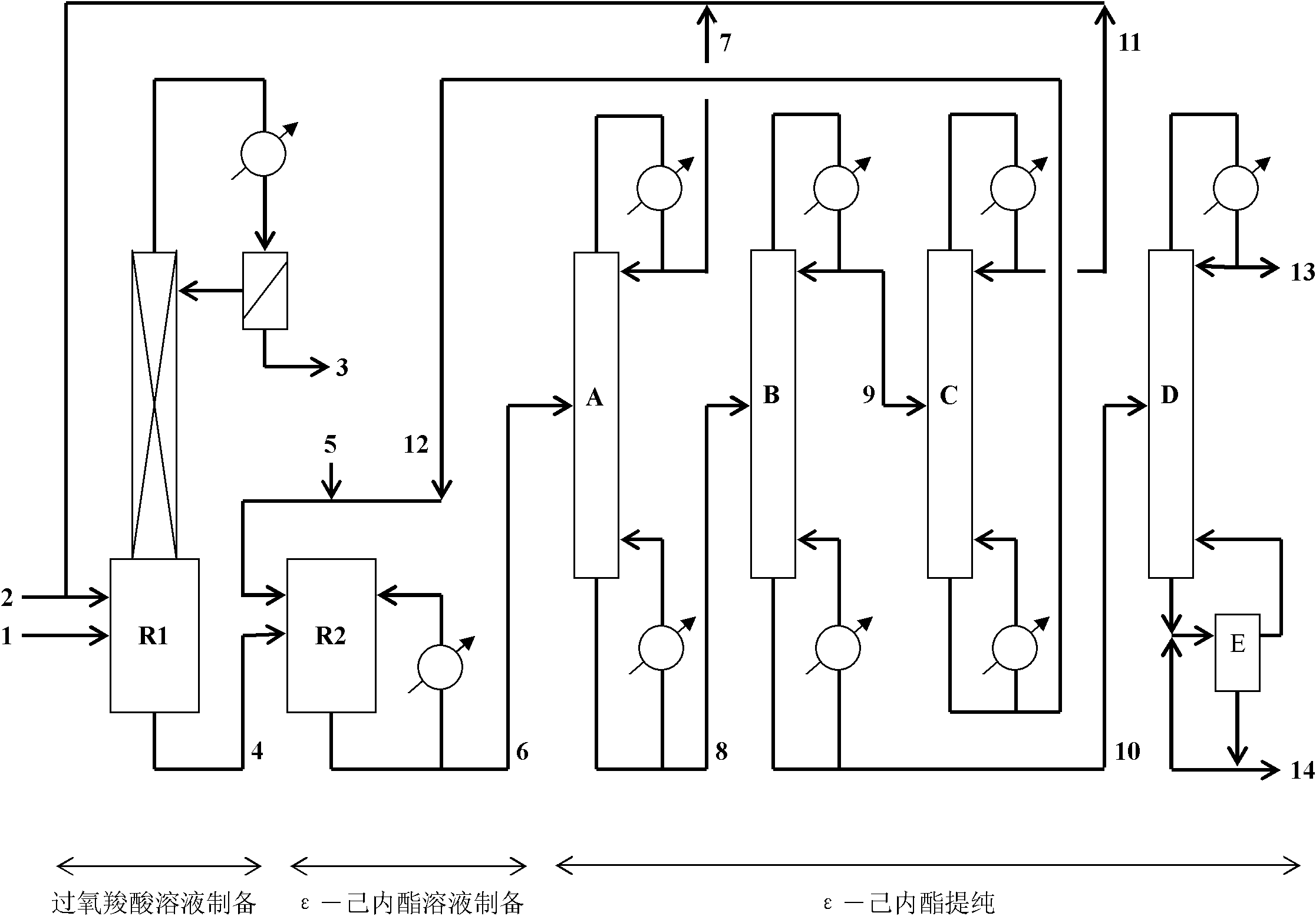
[0043] Preparation of peracetic acid solution
[0044] First put 100kg of perfluorosulfonic acid resin into a volume of 2m 3 In the reactor R1, 600kg acetic acid, 440kg ethyl acetate and 1kg tributyl phosphate are charged into R1 from the input pipe 2.
[0045] The reactor has a packed distillation column and a reflux condenser with a settling tank. Stir this solution at about 20kPa (absolute pressure), heat it to about 40°C with steam, and add a total amount of 220kg of 50% (by weight) hydrogen peroxide solution through pipeline 1 into R1. Control the temperature of R1 to about 40-45°C, return the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser to R1, and the water phase is continuously discharged from the pipe 3 and recovered by the organic solvent The unit recovers the organic solvent therein and refluxes to R1. Acetic acid and hydrogen peroxide have been reacted until the water phase is basically not separated...
Embodiment 2
[0054] Preparation of peroxypropionic acid solution
[0055] 100kg of strongly acidic cationic resin (D001 type, produced by Changsha Dayu Chemical Co., Ltd.) is loaded in advance with a volume of 2m 3 In the reactor R1, 600kg propionic acid, 400kg ethyl propionate and 1kg tributyl phosphate are charged into R1 from the input pipe 2. Stir this solution at about 10kPa (absolute pressure), heat it to about 50°C with steam, and add a total amount of 220kg of 50% (by weight) hydrogen peroxide solution through pipeline 1 into R1. Control the temperature of R1 at about 50-55°C, return the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser to R1, and the water phase is continuously discharged from the pipe 3 and recovered by the organic solvent The unit recovers the organic solvent therein and refluxes to R1. Propionic acid and hydrogen peroxide have been reacted until the water phase is basically not separated in the sedime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com