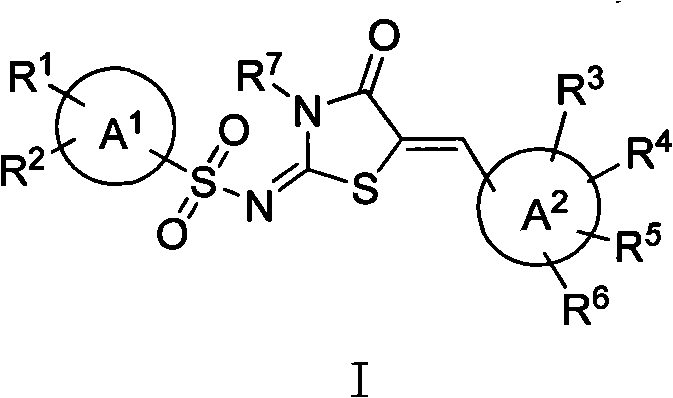
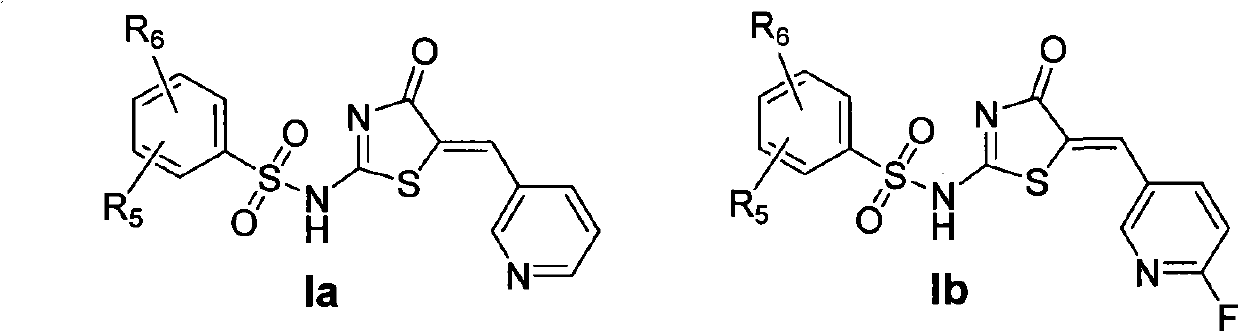
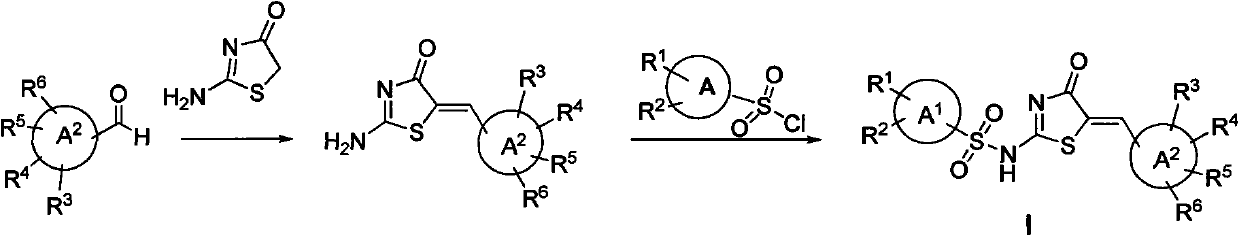
Amion thiazolidone compound, method for preparing same and application thereof in preparing antitumor drugs
A technology of aminothiazolidinone and compound, applied in the field of medicinal chemistry, can solve the problem that there is still no effective inhibition of Plk1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 intermediate 2-amino-5-(3-pyridine methylene)-4(5H)-thiazolone
[0063] To a suspension of 13.4 g (125 mmol) of 3-pyridinecarbaldehyde and 9.67 g (83.36 mmol) of thiazolamine in 60 mL of acetic acid was added 5.2 g (58.4 mmol) of alanine at room temperature. The suspension was heated at 100°C for 4 hours. Then the suspended solid was filtered and the filter cake was washed with a small amount of acetic acid and then washed with water, and dried in vacuum (40°C, 10mmHg) to obtain white 2-amino-5-(3-pyridylmethylene)-4(5H)- Thiazolone solid (16 g, yield: 62.5%).
Embodiment 2
[0064] Example 2 The preparation method of the intermediate 2-amino-5-(3-(5-fluoropyridyl)methylene)-4(5H)-thiazolone is the same as that of Example 1. 3-(5-Fluoropyridine)carbaldehyde was used as starting material.
Embodiment 3
[0065] Example 3 The preparation method of the intermediate 2-amino-5-(3-(2,4-dimethoxypyridine)methylene)-4(5H)-thiazolone is the same as that of Example 1. 3-(2,4-dimethoxypyridine)carbaldehyde was used as starting material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com