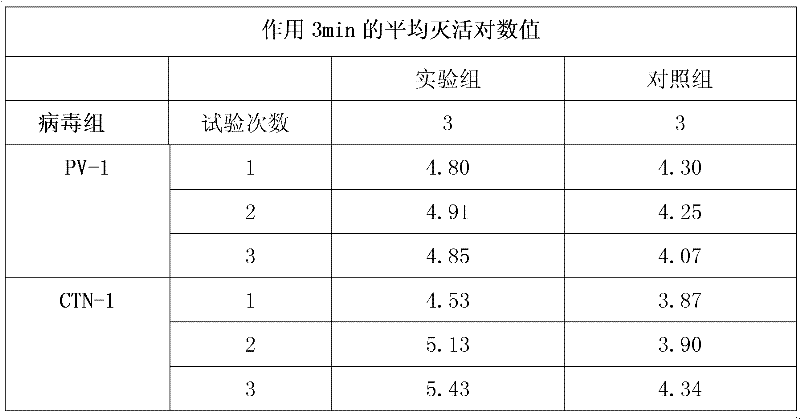
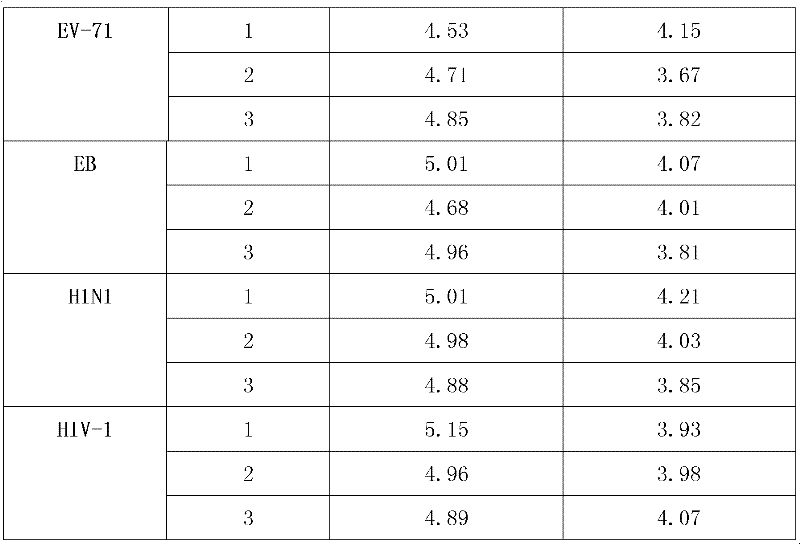
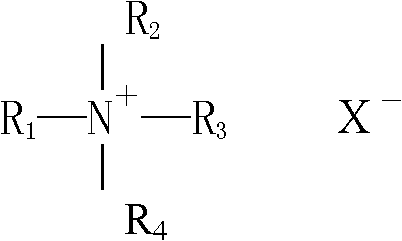I, II and III medical equipment products acting on skin, mucosa and wound for inactivating virus
A technology for medical devices and viruses, applied in the field of skin and mucous membrane medical devices, can solve problems such as little virus inactivation, and achieve the effects of rapid and efficient inactivation, small side effects and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1, Class I, II, and III medical devices according to the present invention
[0100] Class I, II, and III medical devices described in the present invention are processed from the following components:
[0101] Compound quaternary ammonium salt, 0.10%
[0102] Chlorhexidine acetate, 0.15%
[0103] Phthalaldehyde, 0.01%
[0104] Povidone-iodine or other colorless iodine, 0.01%
[0105] Synergist, 0.01%
[0106] Surfactant, 1.00%
[0107] Chlorine dioxide, 0.01%
[0108] Chelating agent, 1.00mg / L
[0109] Stabilizer, 0.50mg / L
[0110] Fragrance, 0.01%
[0111] PH regulator is used according to the actual situation (<10.00%)
[0112] The above-mentioned liquid and liquid dressing formula include (wound surface, hemostasis, burn, antibacterial, nursing, repairing, cleaning) film, paste, ointment, liquid, agent, gel, material, liquid, dressing and so on.
[0113] Disposable medical cotton swabs (wet wipes, towels, gauze, cotton swabs, non-woven fabrics, p...
Embodiment 2、I、II
[0122] Example 2, Class I, II, III medical devices
[0123] The disinfection class I, II, and III medical devices described in the present invention are processed from the following components:
[0124] Compound quaternary ammonium salt, 0.18%
[0125] Chlorhexidine acetate, 0.19%
[0126] Phthalaldehyde, 0.05%
[0127] Povidone iodine or other colorless iodine, 2.00%
[0128] Synergist, 0.05%
[0129] Surfactant, 5.00%
[0130] Chlorine dioxide, 0.2%
[0131] Chelating agent, 5.00mg / L
[0132] Stabilizer, 49.75mg / L
[0133] Fragrance, 5.00%
[0134] PH regulator is used according to the actual situation (<10.00%)
[0135] The above-mentioned liquid and liquid dressing formulations include (wound surface, hemostasis, burn, antibacterial, nursing, repairing, cleaning) film, paste, ointment, liquid, agent, gel, material, liquid, dressing and so on.
[0136] Disposable medical cotton swabs (wet wipes, towels, gauze, cotton swabs, non-woven fabrics, polymer materials). ...
Embodiment 3、I、II
[0145] Example 3, Class I, II, III medical devices
[0146] The disinfection class I, II, and III medical devices described in the present invention are processed from the following components:
[0147] Compound quaternary ammonium salt, 0.18%
[0148] Chlorhexidine acetate, 0.19%
[0149] Phthalaldehyde, 0.08%
[0150] Povidone iodine or other colorless iodine, 3.00%
[0151] Synergist, 0.08%
[0152] Surfactant, 8.00%
[0153] Chlorine dioxide, 0.30%
[0154] Chelating agent, 8.00mg / L
[0155] Stabilizer, 75.00mg / L
[0156] Fragrance, 8.00%
[0157] PH regulator is used according to the actual situation (<10.00%)
[0158] The above-mentioned liquid and liquid dressing formulations include (wound surface, hemostasis, burn, antibacterial, nursing, repairing, cleaning) film, paste, ointment, liquid, agent, gel, material, liquid, dressing and so on.
[0159] Disposable medical cotton swabs (wet wipes, towels, gauze, cotton swabs, non-woven fabrics, polymer materials). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com