Synthesis method of tamibarotene
A technology for the synthesis of tamibarotene, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of cumbersome operation, serious environmental pollution, and high environmental cost, and avoid toxic gases and Effects of acid waste, reduction of environmental costs, and avoidance of high-pollution links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will now be described in further detail in conjunction with the accompanying drawings and embodiments.
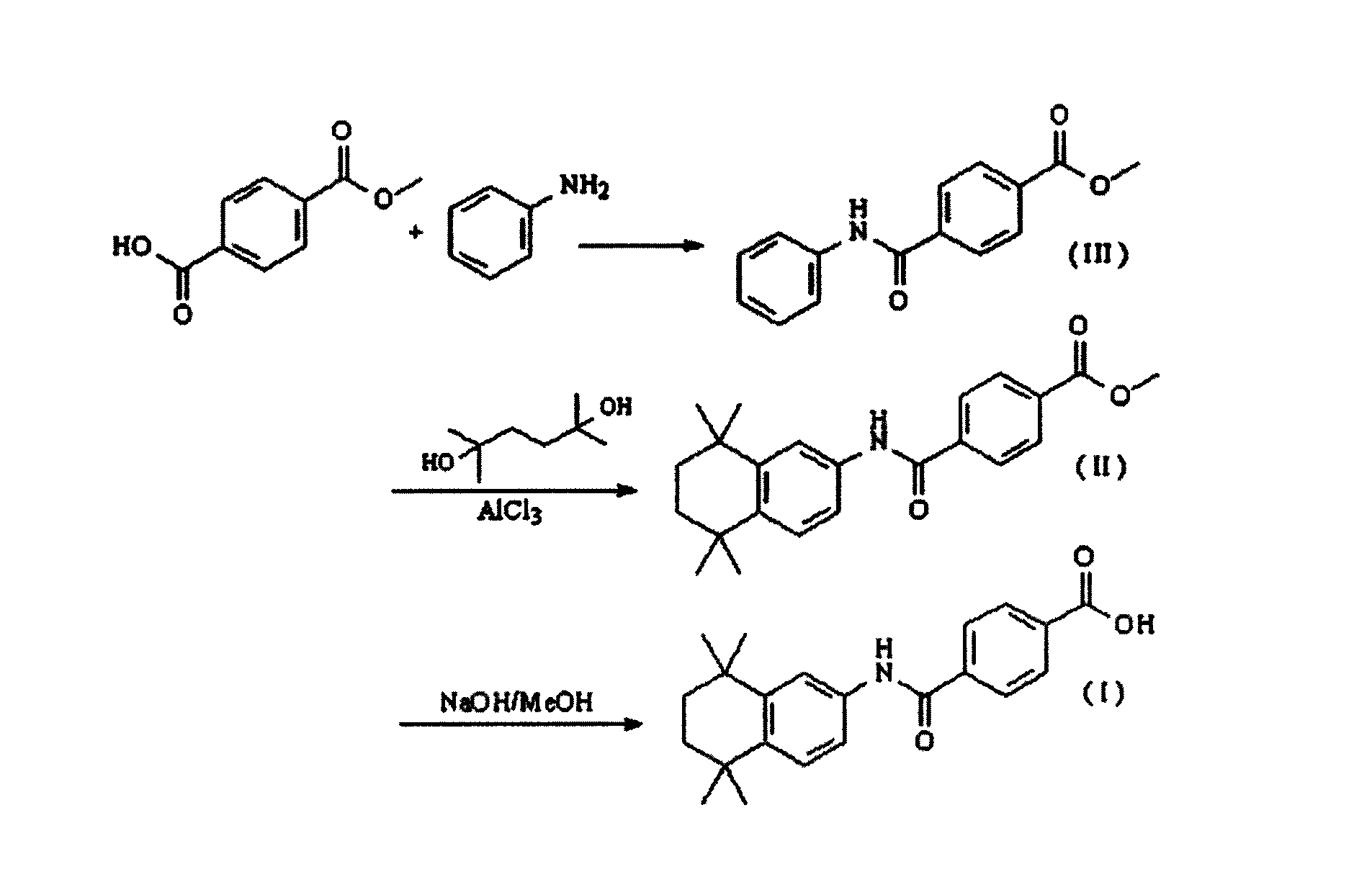
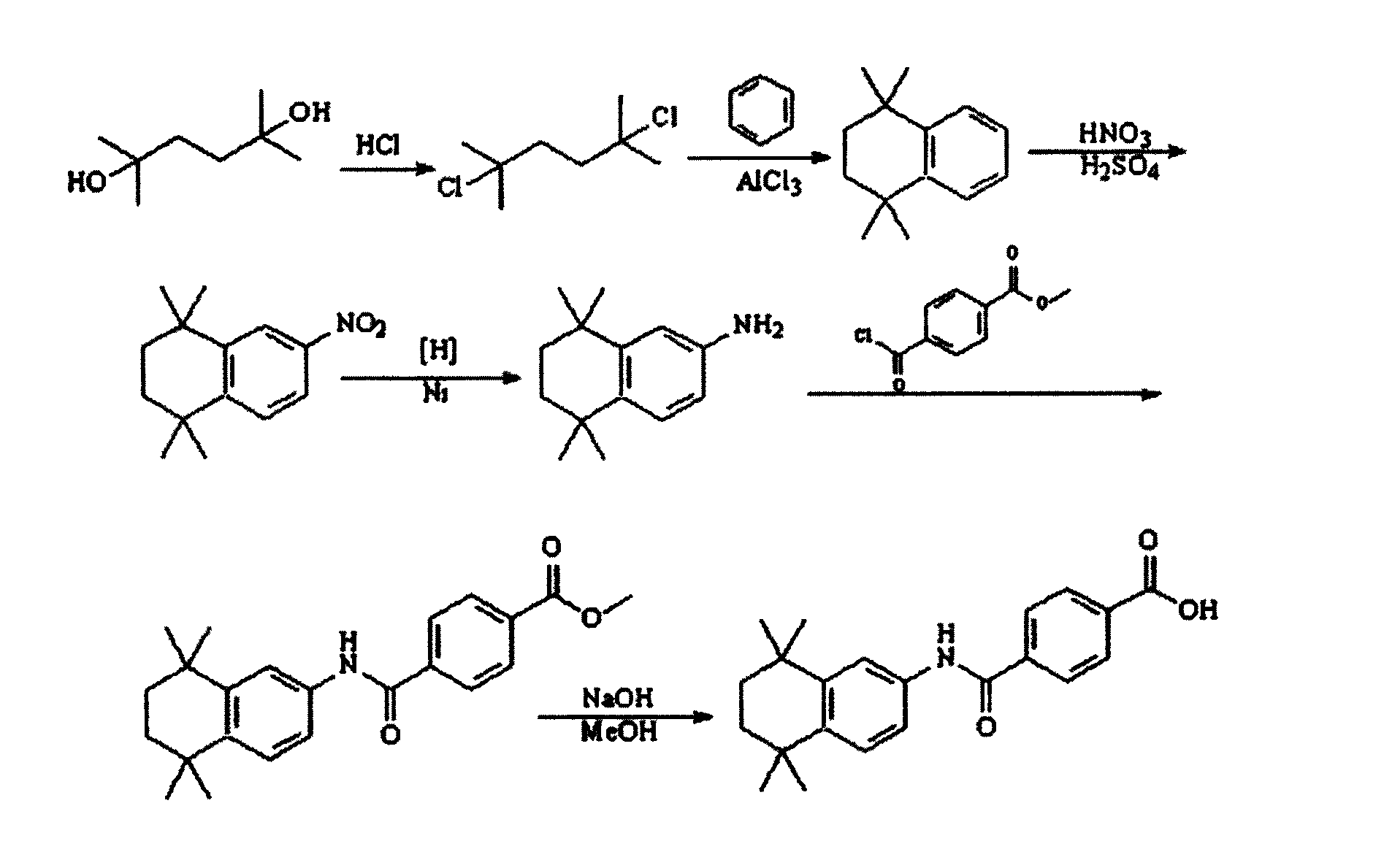
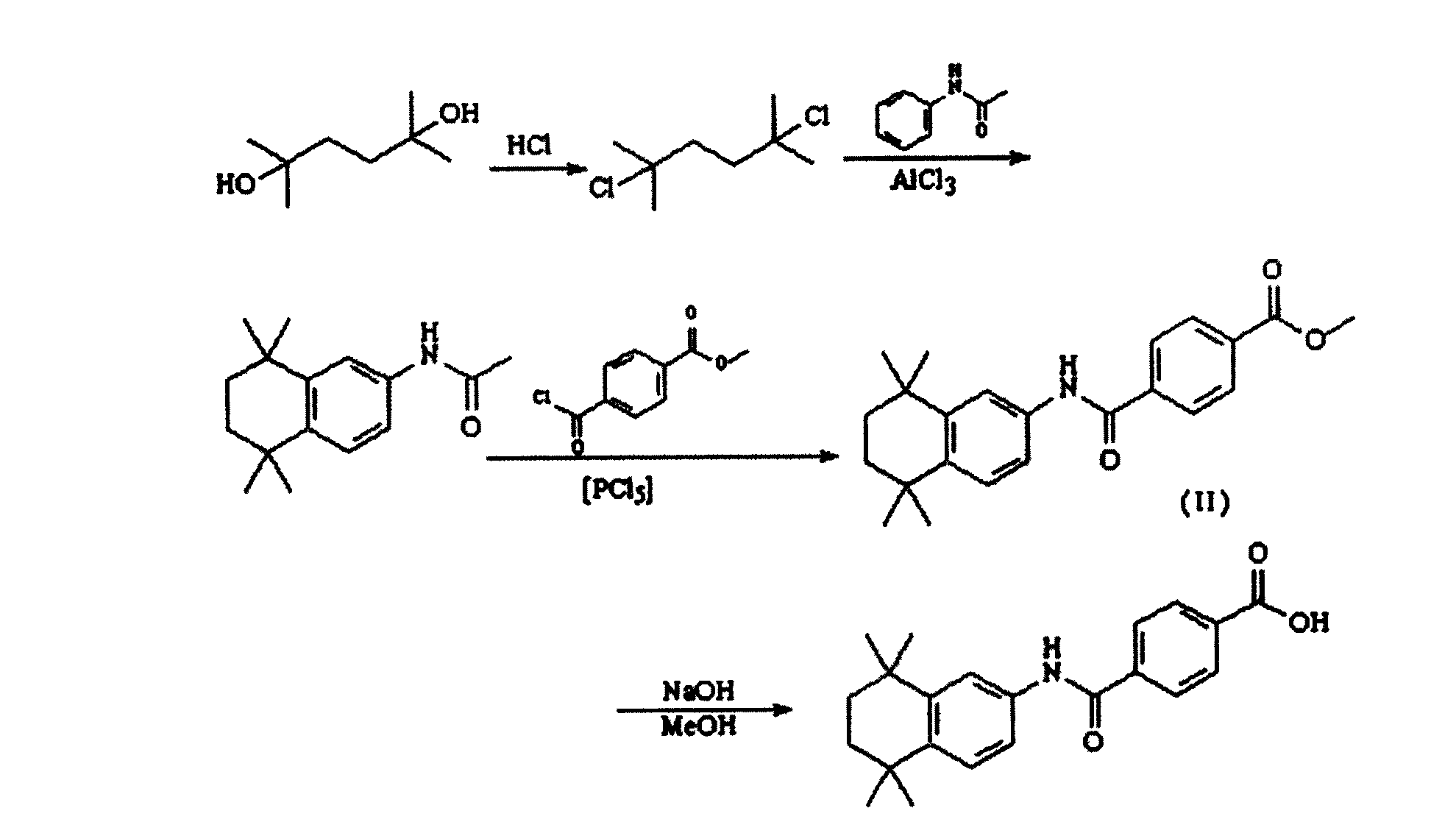
[0030] see figure 1 , is the synthetic route map of the present invention.
[0031] The synthetic method of tamibarotene (I) of the present invention, comprises the steps:
[0032] 1) using aniline and monomethyl terephthalate as raw materials to synthesize methyl p-anicarbamoylbenzoate (III);
[0033]
[0034] 2) Under anhydrous and nitrogen protection, intermediate III is cyclized with 2,5-dimethyl-2,5-hexanediol at low temperature to obtain intermediate II;
[0035]
[0036] 3) The intermediate II is hydrolyzed to obtain the target product-tamibarotene (I).
[0037]
[0038] In the above step 1), DCC / HOBt, DIC / HOBt, HATU, HBTU, etc. are added as condensing agents.
[0039] Above-mentioned steps 1) in, take triethylamine, DIEA as acid-binding agent;
[0040] Above-mentioned step 2) in, add halogen acid, as AlCl 3 as a catalyst....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com