Camptothecin derivative and preparation method and application thereof
A technology of camptothecin and derivatives, applied in the field of medicinal chemistry and therapeutics, which can solve the problems of urinary system and digestive system side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
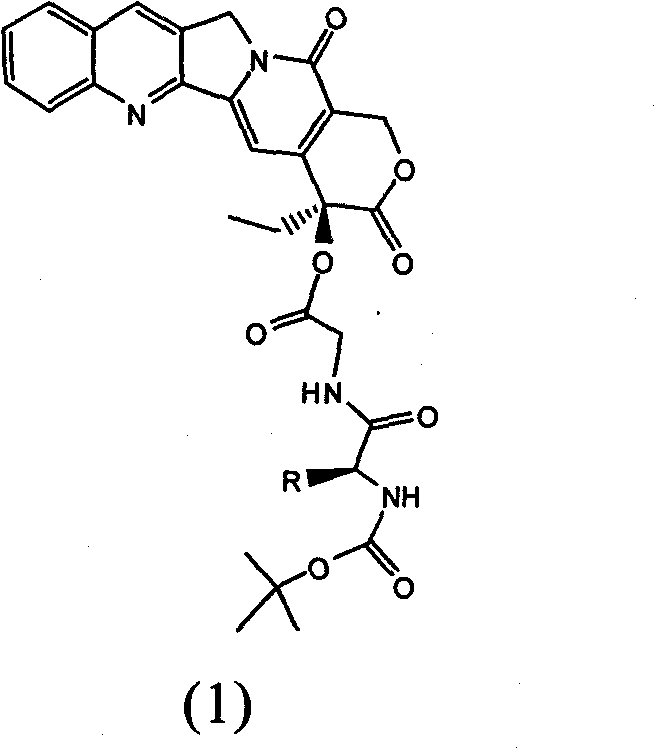
[0058] The preparation method of described 20 (S)-camptothecin derivatives, described organic solvent is dimethyl sulfoxide and N, N-dimethylformamide; Described coupling agent is DCC, CDI and EDC.HCl; The catalyst is pyridine and DMAP.
[0059] A pharmaceutical composition, containing the camptothecin derivative and a pharmacodynamically acceptable carrier.
[0060] The pharmaceutical composition can be tablets, capsules, pills, injections, sustained-release preparations, controlled-release preparations or various particle delivery systems.
[0061] The application of the compound in the preparation of antitumor drugs.
Embodiment 1
[0062] The preparation of embodiment 1 intermediate II
[0063] Dissolve 2.1 g of N-tert-butoxycarbonylglycine at room temperature in 40 ml of N, N-dimethylformamide, add 1 g of camptothecin, 2 g of DCC and 0.25 g of DMAP under stirring, and react for 12 After 1 hour, the precipitate was removed by filtration, and the filtrate was diluted with 100 milliliters of distilled water to separate out a white precipitate. The precipitate was filtered, washed with water, dried and recrystallized to obtain 860 mg of milky white powder crystal intermediate II (86% yield).
Embodiment 2
[0064] The preparation of embodiment 2 intermediate III
[0065] Dissolve 0.5 g of intermediate II in 20 ml of a mixed solution of dichloromethane and trifluoroacetic acid 1:1 (V:V), stir at room temperature for 1 hour, remove dichloromethane by rotary evaporation, and pour the remaining liquid into 50 mL of distilled water precipitated out. The precipitate was filtered, washed with water and dried. 390 mg of intermediate III was obtained as a milky white solid (yield 78%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com