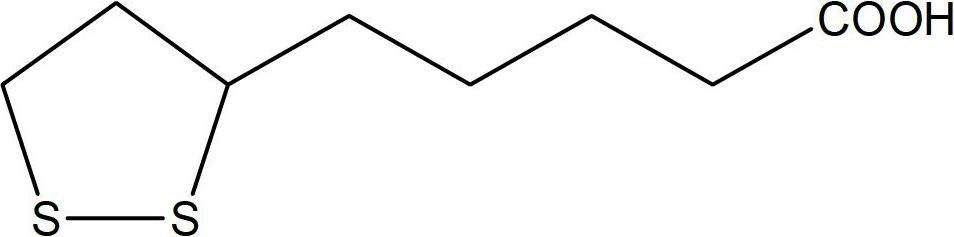
Lipoic acid injection for intravenous administration
A lipoic acid and injection technology, which is applied in the directions of pharmaceutical formulation, drug combination, drug delivery, etc., can solve problems such as being unable to tolerate terminal sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prescription (1000 formulation units):
[0046] Alpha Lipoic Acid 300g
[0047] Meglumine 90g
[0048] Add water for injection to 12000ml
[0049] Preparation process: Add lipoic acid to 11500ml of water for injection, add meglumine during continuous stirring, until the pH value of the solution is 8.02 after clarification, add 6g of activated carbon, stir for 20 minutes, filter and decarbonize with a 0.80μm microporous membrane , add water for injection to 12000ml, filter through a 0.22μm microporous membrane, potting, and autoclave at 115°C for 30min to obtain the product.
Embodiment 2
[0051] Prescription (1000 formulation units):
[0052] Alpha Lipoic Acid 300g
[0053]Meglumine 105g
[0054] Add water for injection to 12000ml
[0055] Preparation process: Add lipoic acid to 11000ml of water for injection, add meglumine during continuous stirring, until the pH value of the solution is 8.13 after clarification, add 5.5g of activated carbon, stir for 20 minutes, filter with a 0.80μm microporous membrane to remove Charcoal, add water for injection to 12000ml, filter through a 0.22μm microporous membrane, potting, and autoclave at 115°C for 30min to obtain the product.
Embodiment 3
[0057] Prescription (1000 formulation units):
[0058] Alpha Lipoic Acid 300g
[0059] Meglumine 120g
[0060] Add water for injection to 12000ml
[0061] Preparation process: Add lipoic acid to 11,500ml of water for injection, add meglumine during continuous stirring, until the pH value of the solution is 8.22 after clarification, add 6g of activated carbon, stir for 20 minutes, filter and decarbonize with a 0.80μm microporous membrane filter , add water for injection to 12000ml, filter through a 0.22μm microporous membrane, potting, and autoclave at 115°C for 30min to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com