Imidazole diphosphonie acid compound and pharmaceutically-acceptable salt and medicinal application thereof
A technology of imidazole bisphosphonic acid and compounds, which is applied in the field of preparation of drugs for the treatment of metabolic bone diseases, can solve problems such as major side effects, and achieve the effects of inhibiting angiogenesis, inhibiting bone resorption, and inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
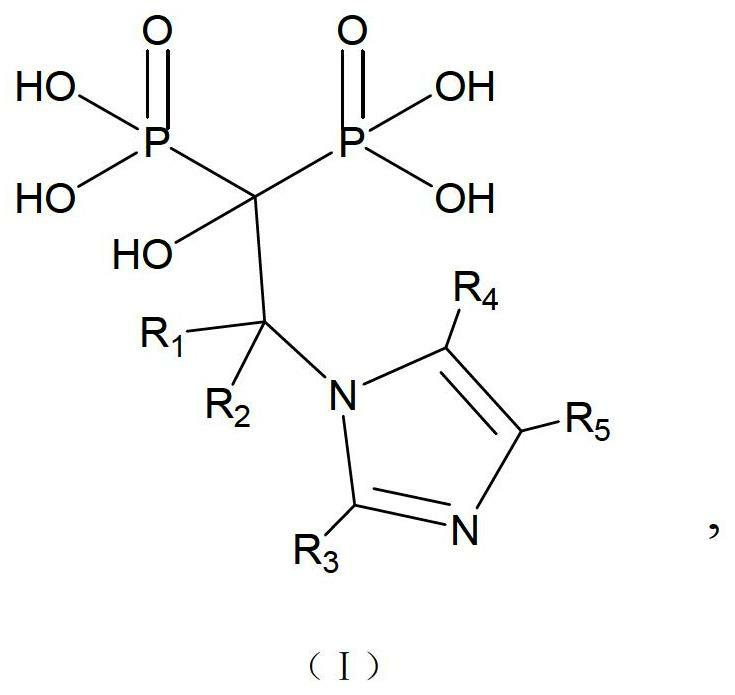
[0044] An imidazole bisphosphonic acid compound, the chemical name is: 1-hydroxy-2-substituted-2-imidazolyl-ethylidene-1,1-bisphosphonic acid, and its structural formula is as follows:
[0045]
[0046] In the above formula, R 1 =C 1 ~C 6 Alkyl, phenyl, or H, Me, F, Br, Cl, OR 3 (R 3 =C 1 ~C 4 Alkyl) and other substituted phenyl groups.
[0047] The above-mentioned imidazole bisphosphonic acid compound (hereinafter referred to as compound 2) is synthesized by the following route:
[0048]
[0049] A specific preparation process is as follows: in a 25ml round neck flask, add 3mmol compound 1, 15mmol H 3 PO 3 and 8ml of toluene, electromagnetically stirred and heated to 80°C, after the solid was completely melted, slowly added 15mmol of phosphorus oxychloride (POCl 3 ), stirred vigorously for 12 to 24 hours, after cooling, removed the toluene solution, added 6ml 6M hydrochloric acid to the reaction flask, refluxed for 1 hour, after cooling, removed the solvent by ...
Embodiment 2
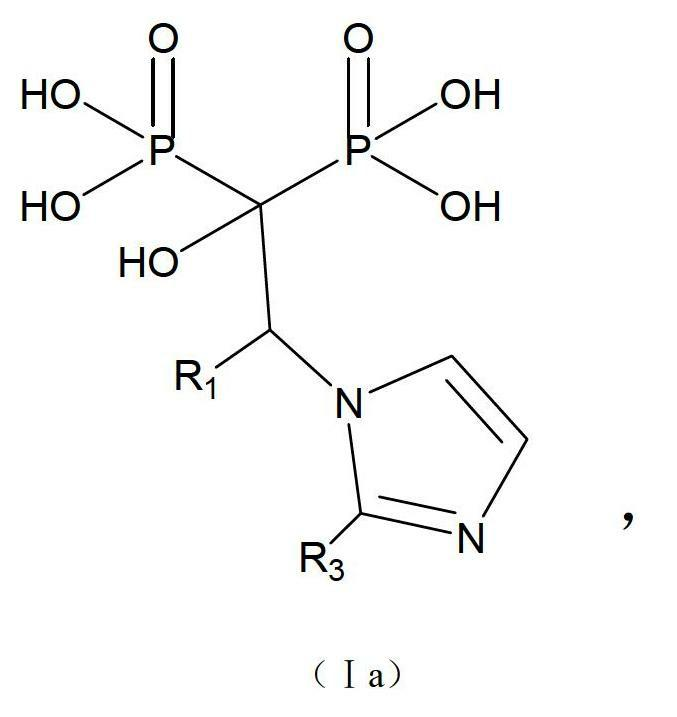
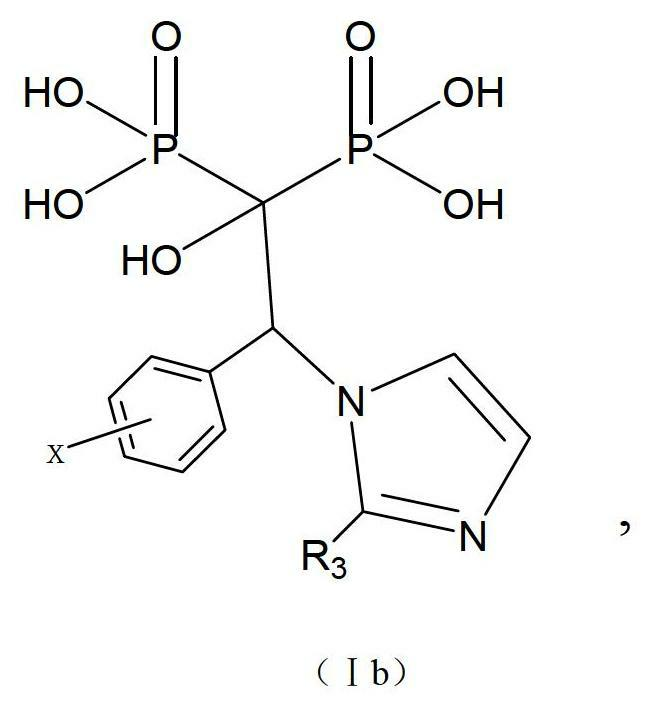
[0068] An imidazole bisphosphonic acid compound, its chemical name is: 1-hydroxy-2-substituted-2-benzimidazolyl-ethylidene-1,1-bisphosphonic acid, the structural formula is as follows:
[0069]
[0070] R 1 =H,C 1 ~C 6 Alkyl or substituted phenyl; Y=H, Me, F, Cl, Br, OR 1 (R 1 =C 1 ~C 4 Alkyl); the above-mentioned imidazole bisphosphonic acid compound (hereinafter referred to as compound 4) is synthesized by the following route:
[0071]
[0072] A specific preparation process is as follows: in a 25ml round neck flask, add 3mmol compound 3, 15mmol H 3 PO 3 Electromagnetically stir with 8ml of toluene and heat to 80°C. After the solid is completely melted, slowly add 15mmol of phosphorus oxychloride, and stir vigorously for 12-24 hours. After cooling, remove the toluene solution and add 6ml of 6M hydrochloric acid to the reaction flask , refluxed for 1 hour, after cooling, the solvent was removed by rotary evaporation, and an appropriate amount of isopropanol was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com