VHH (variable domain of heavy chain of heavy-chain antibody) antibody gene derived from anti-CyPA (CyclophilinA) animal of family Camelidae, encoded polypeptide, and application thereof
A gene and animal technology, applied in the direction of anti-animal/human immunoglobulin, plant gene improvement, application, etc., can solve problems such as antibody drugs that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] (1) Screening of anti-CypA heavy chain VHH antibody A1
[0018] 1. Immunity of alpacas
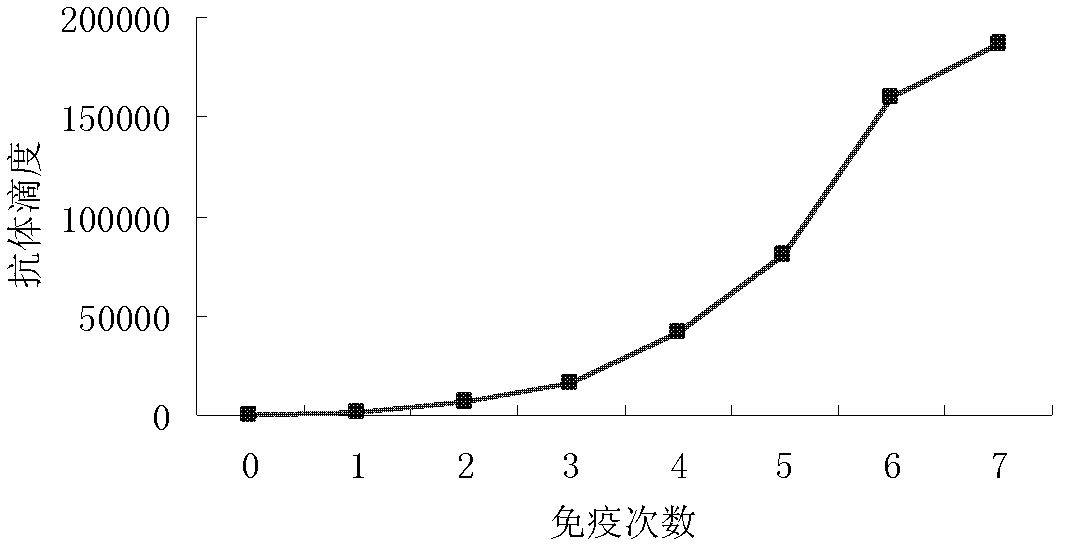
[0019] Two alpacas were immunized with CypA, and the immunization scheme was as follows: Alpaca antigen dosage for immunization: 0.5mg, 0.5mg, 1.0mg, 1.0mg, 2.0mg, 4.0mg, 4.0mg. The interval between immunizations was 2 weeks. Method: The first immunization was to grind and mix the antigen plus Freud's complete adjuvant, and inject it subcutaneously at multiple points. Subsequent immunization, antigen plus Freud's incomplete adjuvant, multi-point subcutaneous injection, 0.5ml / point, 4 points / time. Take a small amount of blood before each immunization, and use the ELISA method to detect the titer of anti-CypA antibody in alpaca blood, such as figure 1 As shown, after 7 times of immunization, the antibody titer in the blood of one alpaca reached 1:186,000.
[0020] 2. Construction of anti-cyclophilin A immune phage antibody library
[0021] The peripheral blood of immunized alpaca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com