Application of a kind of ethyl ketone compound in preparation of medicine for treating inflammation
A compound, the technology of ethyl ketone, which is applied in the field of PI3Kγ-specific inhibitors, can solve the problems of limited drug effects in inflammatory diseases, and achieve the effect of inhibiting chemotaxis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
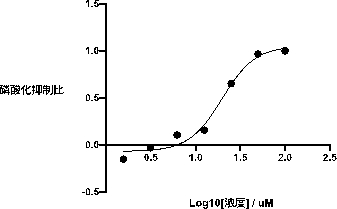
[0027] PI3Kγ blank group: use a 384-well plate, and set up 3 parallel experimental groups. Configure PI3K reaction system 5 μL, each well
[0029] PI3Kγ experimental group: use a 384-well plate, and set up 3 parallel experimental groups. Compound 1‑(5‑(3‑
[0034]
Embodiment 2
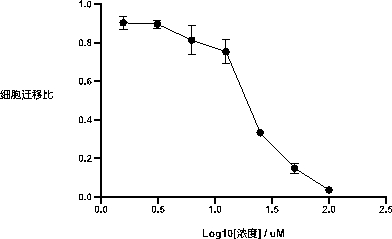
[0036] Using the RAW264.7 cells under the action of the chemokine C5a as a cell model for the specific activation of the PI3Kγ pathway,
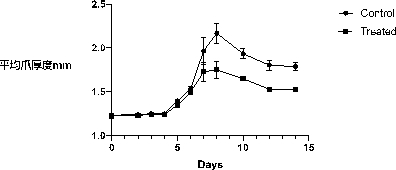
Embodiment 3
[0040] Leukocytes, especially neutrophils and monocytes, are exposed to chemokines such as C5a, fMLP, CCL3, CXCL1-3
[0041] The experiment is divided into control group and administration group. First put the inner side of the Transwell chamber into a 24-well plate, and add the inner chamber to the 24-well plate.
[0042] The results are shown in Figure 2. The results show that macrophage RAW264.7 can produce chemotaxis under the action of chemokines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com