Pharmaceutical composition for the treatment of bladder disorders
A composition and drug technology, which can be used in drug combinations, sexual diseases, urinary system diseases, etc., and can solve problems such as deterioration and serious bladder inflammation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
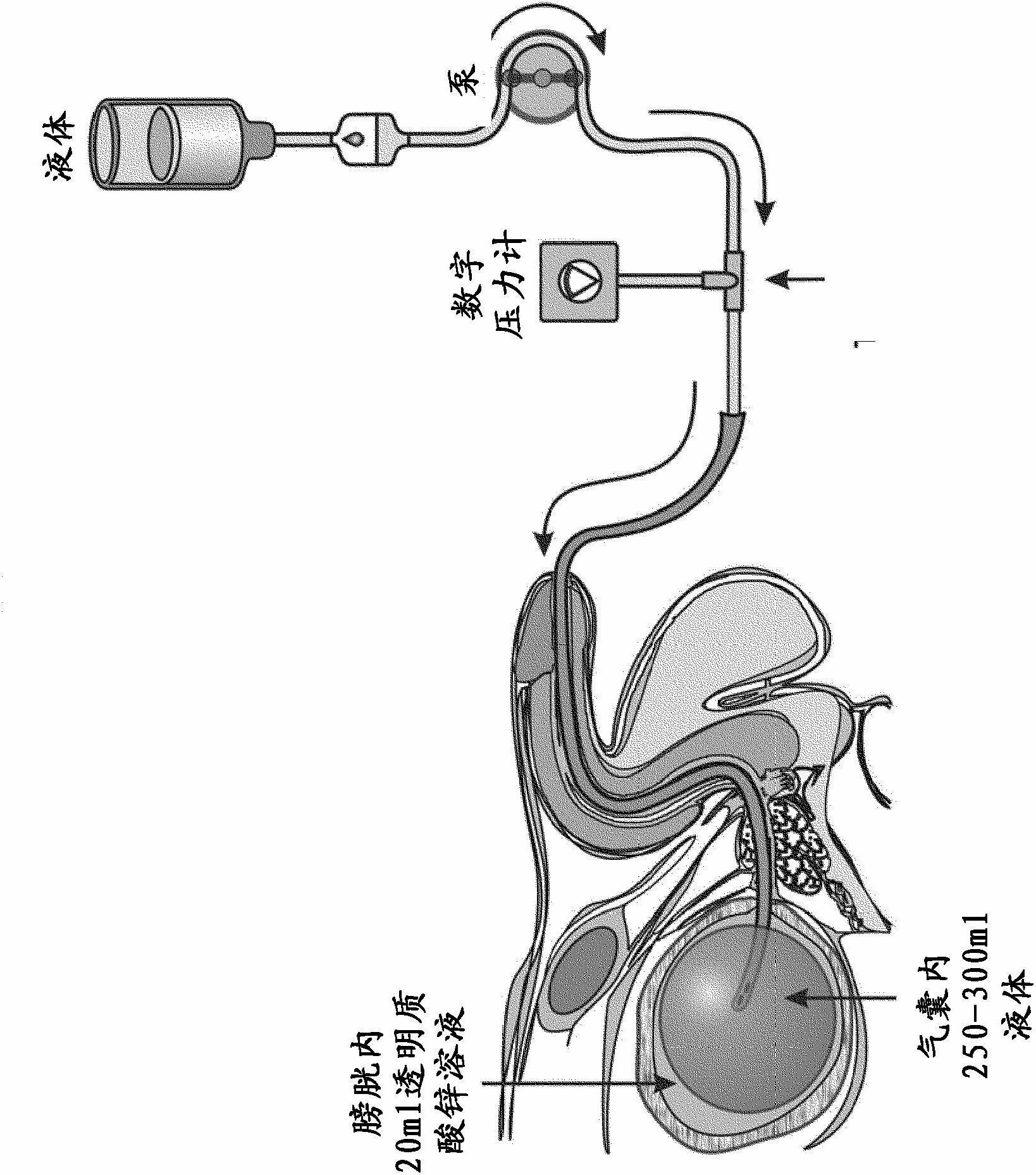
[0044] Weigh 0.20 mg of sodium hyaluronate (Reag.Ph.Eur.) in a 100 ml flask, then add 5.0 ml of distilled distilled water (water for injection, pyrogen-free, sterile) at a concentration of 0.10 mol / l Zinc solution, followed by double distilled water to bring the volume up to 50 ml. 23.5 ml of a 1.00 mol / l sorbitol solution (prepared with double distilled water) were added. Then, the volume was brought up to 100 ml with double distilled water. Finally, the solution was filtered with a membrane filter.
[0045] animal model test
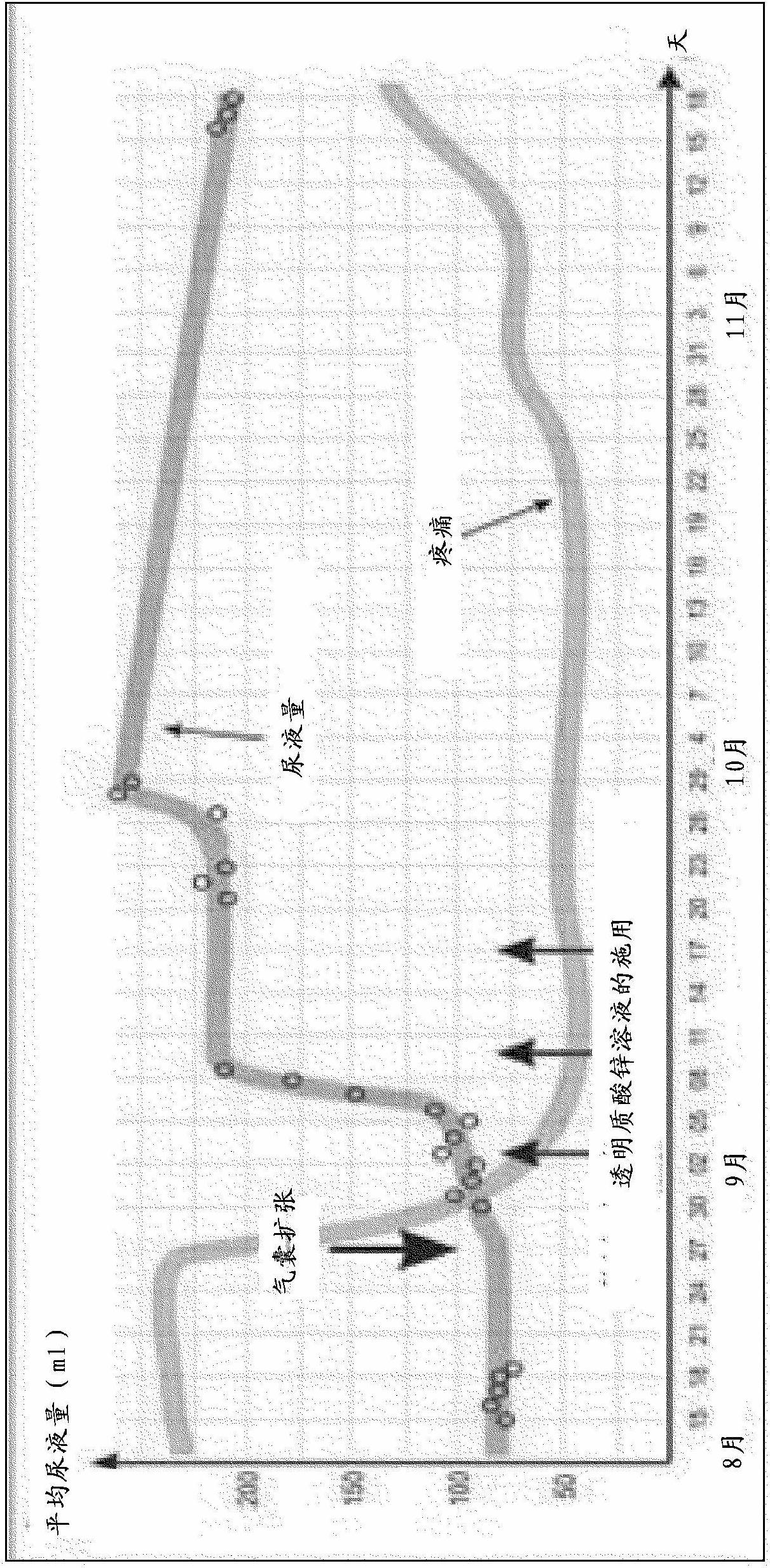
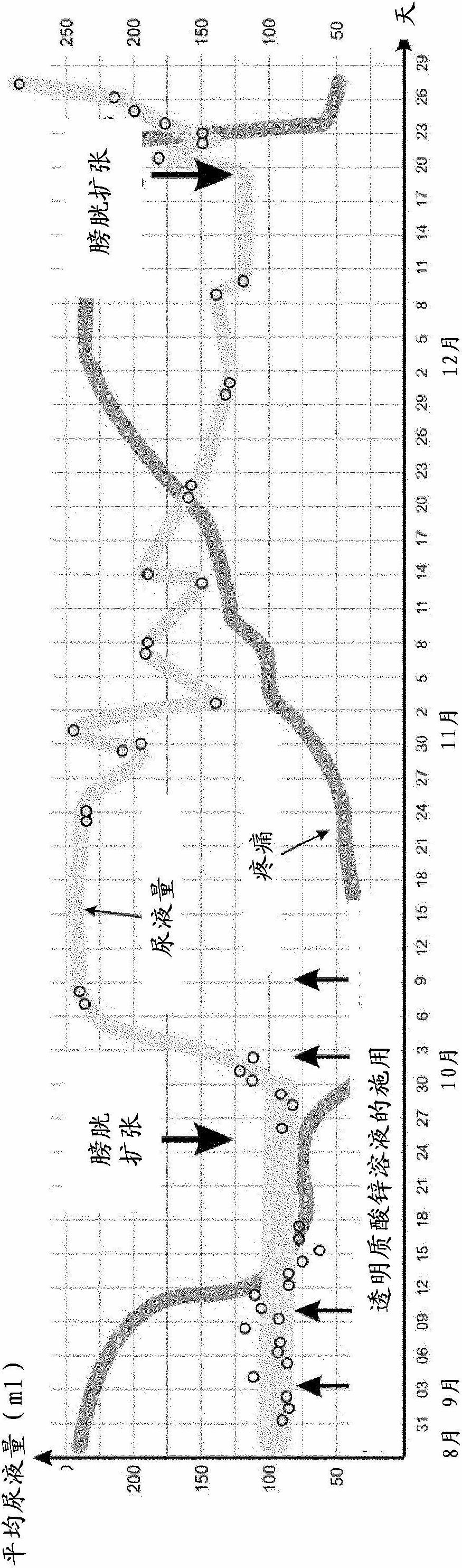
[0046] Two series of trials were designed to evaluate the efficacy of a zinc hyaluronate solution in regeneration of the bladder wall. In the first series of trials, the experimental pathological process served as a model for the changes that occur in the bladder wall in interstitial cystitis. The second series of tests simulates the acute inflammatory process of the bladder.
Embodiment 2
[0048] Interstitial cystitis model
[0049] The test was done with 20 white female rats. Simulates an experimental pathological process similar to that occurring on the bladder wall in interstitial cystitis. Cryodestruction of the bladder. A cotton swab impregnated with liquid nitrogen is introduced into the bladder and left for 20 seconds to induce interstitial cystitis. Animals were then selected into 3 experimental groups, respectively.
[0050] Test Group 1: 48 hours after freezing impairment, the rats received only one treatment, 1 ml of zinc hyaluronate solution (according to Example 1) was introduced into the bladder of the rats and left for 30 minutes.
[0051] Test Group 2: Animals received the same treatment as Group 1 animals, but 3 times (done in 3 consecutive days).
[0052] Experimental Group 3: 3 control animals received no treatment after freezing impairment.
[0053] Subsequently, the rats were sacrificed with an overdose of sodium thiopental and the blad...
Embodiment 3
[0057] Acute Bacterial Cystitis Model
[0058] The test was done with 16 white female rats. Acute inflammation of the bladder was injected (under pressure) with 1.0ml Escherichia coli culture solution (the concentration of bacteria solution was 10 6 CFU / ml) to induce.
[0059] Animals were then selected into 3 experimental groups:
[0060] Test Group 1: 48 hours after the injection of the E. coli culture solution, the rats received only one treatment, 1 ml of zinc hyaluronate solution (according to Example 1) was introduced into the bladder of the rats and kept for 30 minutes.
[0061] Test Group 2: Rats received the same treatment as Group 1 animals, but 3 times (done in 3 consecutive days).
[0062] Test group 3: 3 control animals received no treatment after injection of E. coli culture solution.
[0063] Subsequently, three groups of rats were sacrificed by overdose of thiopental and their bladders were excised. For histological studies, the above bladder samples were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com