Screw epoxidation indole heterocyclic compound as well as synthetic method and purpose thereof
A technology of heterocyclic compounds and indoles, which is applied in the field of spirocyclic indole heterocyclic compounds, can solve problems such as the unstable structure of chrysanthemum-like antifeedants, and achieve improved antifeedant activity, stable structure, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
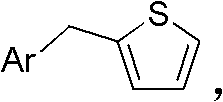
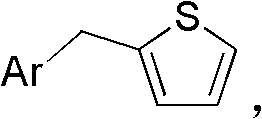
[0060] (1). Add 100mmol of benzyl bromide, 120mmol of thiophene boronic acid, 1.2mmol of Pd(PPh 3 ) 4 , 200mL ethylene glycol dimethyl ether and 100mL water, 200mmol of Na 2 CO 3 , heated to reflux at 100°C under the protection of nitrogen, reacted for 5 hours, removed the solvent under reduced pressure, and isolated to obtain Yield 90%.
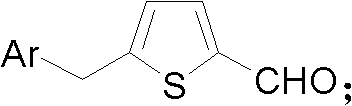
[0061] (2). Add 80mmol of DMF and 100mL of dichloromethane into a 500mL single-necked flask, stir for half an hour, then slowly add 80mmol of POCl dropwise under ice bath conditions 3 , stirred at room temperature (25°C) for half an hour, then slowly added 20 mmol dropwise in ice bath Then placed at room temperature (25°C) and stirred for half an hour, heated to reflux at 50°C for 12 hours, added a strong base to alkalinize the system until the pH of the system was 7, and separated the liquid product to obtain, Yield about 95%.
[0062] (3). Add 5g to a 500mL single-necked flask 10g KMnO 4 , a mixed solution of 200 mL of acetone ...
Embodiment 2
[0072] The difference between this embodiment and embodiment 1 is: the reactant of step (5) for The compound obtained is: Its physical constants are:
[0073] 1 H NMR (400MHz, CDCl 3 )δ=7.46-7.44(m, 2H), 7.33-7.26(m, 2H), 7.20-7.17(m, 1H), 6.87(s, 1H), 6.73(s, 1H), 6.69(d, J= 6.0Hz, 1H), 6.48(s, 1H), 5.80(d, J=6.0Hz, 1H), 3.93(s, 3H), 3.82(s, 3H), 3.78(q, J=7.2Hz, 2H) , 1.31(t, J=7.2Hz, 3H);
[0074] 13 C NMR (100MHz, CDCl 3 )δ174.6, 150.8, 145.8, 142.8, 137.3, 136.7, 135.8, 132.4, 128.5, 128.2, 126.8, 121.0, 119.5, 109.8, 94.5, 56.8, 56.6, 35.6, 30.1, 12.9.
[0075] MS(ESI): m / z=280(M+H) + .
[0076] Elemental Analysis: C 22 h 21 NO 3 Calculated for S: C, 69.63; H, 5.58; N, 3.69; O, 12.65; S, 8.45 Found: C, 69.52; H, 5.71; N, 3.80; S, 8.59.
Embodiment 3
[0078] The difference between this embodiment and embodiment 1 is that the reactant of step (5) for The compound obtained is: Its physical constants are:
[0079]1 H NMR (400MHz, Acetone) δ=7.47-7.45(m, 2H), 7.38-7.34(m, 2H), 7.21-7.17(m, 1H), 6.73(s, 1H), 6.73(d, J=6.0 Hz, 1H), 6.37(s, 1H), 6.29(s, 1H), 5.88(d, J=6.0Hz, 1H), 3.86(s, 3H), 3.76(q, J=7.2Hz, 2H), 3.75(s, 3H), 1.22(t, J=7.2Hz, 3H);
[0080] 13 C NMR (100MHz, Acetone) δ174.76, 164.20, 158.86, 145.52, 145.35, 138.32, 137.76, 133.62, 129.33, 128.75, 127.17, 119.64, 105.97, 93.56, 89.86, 604.13, 56.1
[0081] MS(ESI): m / z=380(M+H) + .
[0082] Elemental Analysis: C 22 h 21 NO 3 Calculated for S: C, 69.63; H, 5.58; N, 3.69; O, 12.65; S, 8.45 Found: C, 69.82; H, 5.69; N, 3.77; S, 8.21.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com