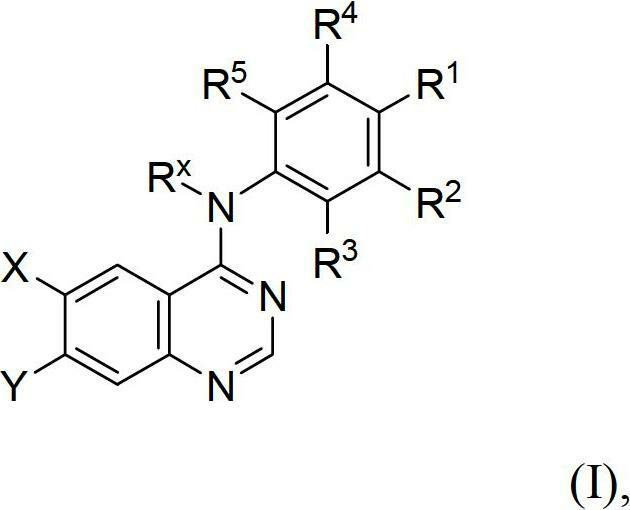
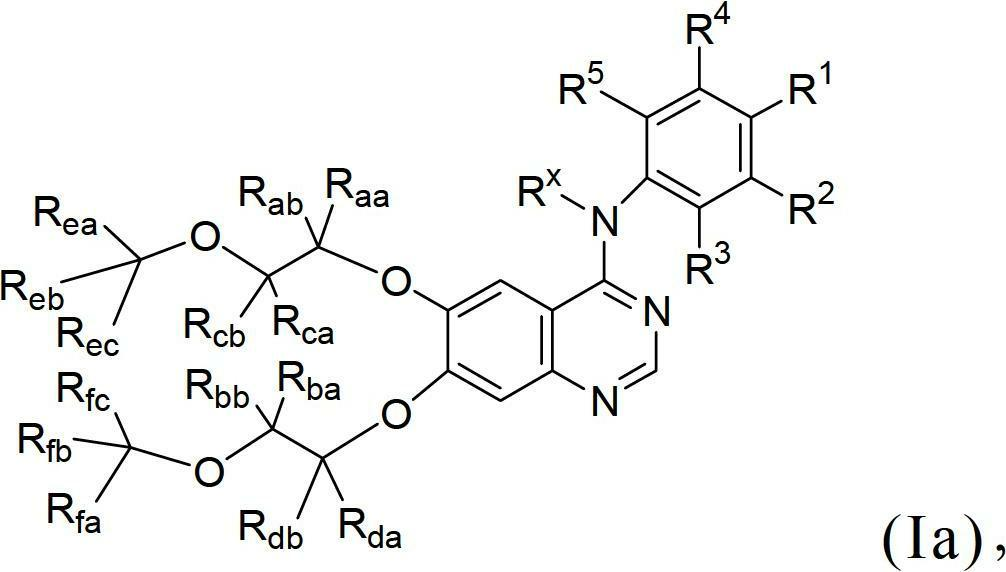
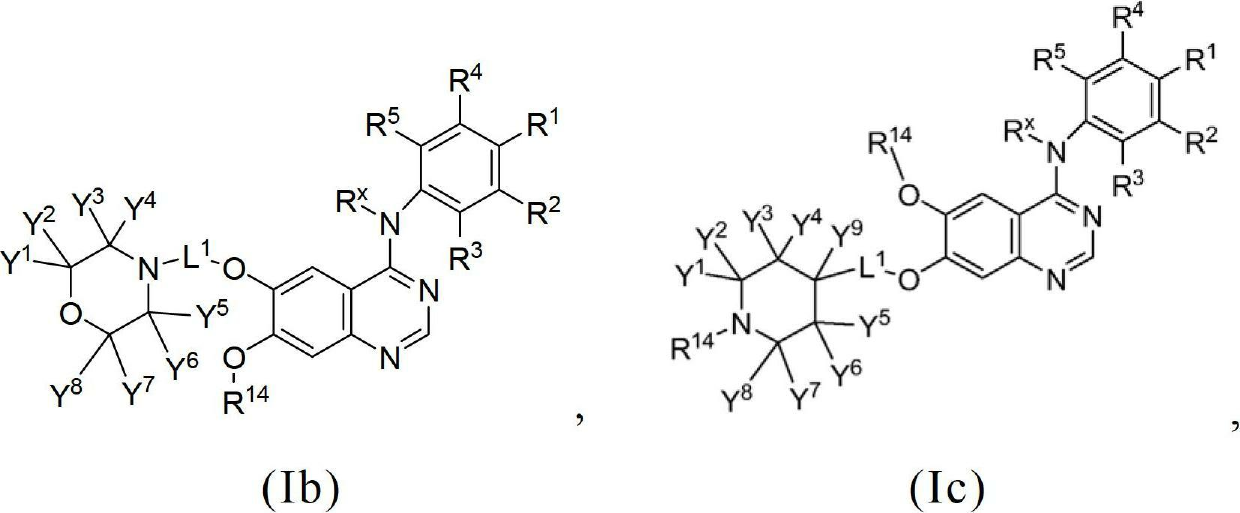
Novel quinazoline derivatives
A compound, prodrug technology, applied in the field of new quinazoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0581] (S,E)-N-(4-(3-Chloro-4-fluoroanilino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yl)-4-(D 6 -Dimethylamino)but-2-enamide
[0582]
[0583] N-(3-Chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine (2).
[0584] 230 mg of 1 (1.0 mmol) and 146 mg of 3-chloro-4-fluoroaniline (1.0 equiv) were suspended in 5 mL of isopropanol and heated at reflux for 4 hours. The mixture was cooled to room temperature and the solvent was evaporated under vacuum to give the crude product. The crude product was dissolved in dichloromethane and basified by 10% sodium hydroxide solution. The organic layer was separated and dried to give pure product 2 as a yellow solid in 100% yield. HPLC-MS: m / z: 337[M+1] + .
[0585] (S)-N-(3-Chloro-4-fluorophenyl)-6-nitro-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine (3).
[0586] 200 mg of 2 (0.6 mmol) was dissolved in 5 mL of DMF, 115 mg of potassium trimethylsilanolate (0.9 mmol) was added at room temperature, and stirred at room temperature for...
Embodiment 2
[0592] (S,E)-4-(D 6 -Dimethylamino)-N-(4-(3-ethynylanilino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yl)but-2-enamide
[0593]
[0594] As described in the scheme above, the compound was synthesized with (S)-N-(4-(3-chloro-4-fluoroanilino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yl )-4-(D 6 -Dimethylamino)but-2-enamide was synthesized similarly, except that 3-ethynylaniline was used instead of 3-chloro-4-fluoroaniline. The final product was purified by HPLC to obtain 50 mg of product as a white solid. HPLC-MS: m / z: 464[M+1] + .
Embodiment 3
[0596] (E)-N-(4-(3-chloro-4-fluoroanilino)-7-((1-D)-tetrahydrofuran-3-yloxy)quinazolin-6-yl)-4-( Dimethylamino)but-2-enamide
[0597]
[0598] N-(3-Chloro-4-fluorophenyl)-7-fluoro-6-nitroquinazolin-4-amine (2).
[0599] 230 mg of 1 (1.0 mmol) and 146 mg of 3-chloro-4-fluoroaniline (1.0 equiv) were suspended in 5 mL of isopropanol and heated at reflux for 4 hours. The mixture was cooled to room temperature and the solvent was evaporated under vacuum to give the crude product. The crude product was dissolved in dichloromethane and basified by 10% sodium hydroxide solution. The organic layer was separated and dried to give pure product 2 as a yellow solid. The yield is 100%. HPLC-MS: m / z: 337[M+1] + .
[0600] N-(3-Chloro-4-fluorophenyl)-6-nitro-7-(3-D-tetrahydrofuran-3-yloxy)quinazolin-4-amine (3).
[0601] 200 mg of 2 (0.6 mmol) was dissolved in 5 mL of DMF, 115 mg of potassium trimethylsiliconate (0.9 mmol) was added at room temperature and stirred for 15 min. Then,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com