Application of small-molecule inhibitor ML385 in inhibition of tumor cells PD-L1
A small molecule inhibitor, PD-L1 technology, applied in the field of biomedicine, to achieve the effect of easy mass production, anti-tumor activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] (1) Preparation of protein samples. Prepare lysis buffer (containing 10 μL PMSF and 990 μL Lysis buffer) and place in an ice bath for later use; cells are washed with pre-cooled PBS and then transferred to a sterilized centrifuge tube, and the supernatant is discarded after centrifugation; cells are resuspended in lysis buffer containing PMSF , lysed on ice bath for 30min (shaker); 4°C, high-speed centrifugation for 15min, and completely aspirated the supernatant (protein). After mixing the protein with 5×Loading buffer, pipette at a ratio of 4:1, boil in a metal bath at 95°C for 5 min, and store at -20°C.
[0076] (2) gel electrophoresis and transfer membrane for color development. Boil the protein sample again at 105°C for 5 min; prepare 10% separating gel according to the formula of protein gel, prepare 5% stacking gel for the upper layer, and add 10 μL protein sample to each well; adjust the voltage of the electrophoresis apparatus to 80V, when the bromophenol blue...
Embodiment 1
[0099] To verify the effect of ML385 inhibitor in tumor cells, the selected target cells were breast cancer SUM159 and MDA-MB-231, respectively. First, recover the cells. The specific example is as follows: take out the cryovial from -80°C, directly immerse it in warm water at 37°C, and shake it to thaw as soon as possible (slow freeze and quick-dissolve); take out the cryovial from the 37°C water bath, add 1 mL of Complete medium, mix well; centrifuge at 1000 rpm for 3 min, discard the supernatant; add DMEM medium containing 10% FBS to resuspend the cells, and evenly inoculate in a 60 mm petri dish, 5% CO 2 , 37°C incubator for static culture; replace the culture medium the next day and continue the culture; when the cell density reaches 90%, trypsinize the cells, and determine the number of wells (6-well plate) according to subsequent experiments, and continue to culture; NRF2 Inhibitor treatment: ML385, the concentration of selective treatment was 0, 20, 50, 100 μM, and the...
Embodiment 2
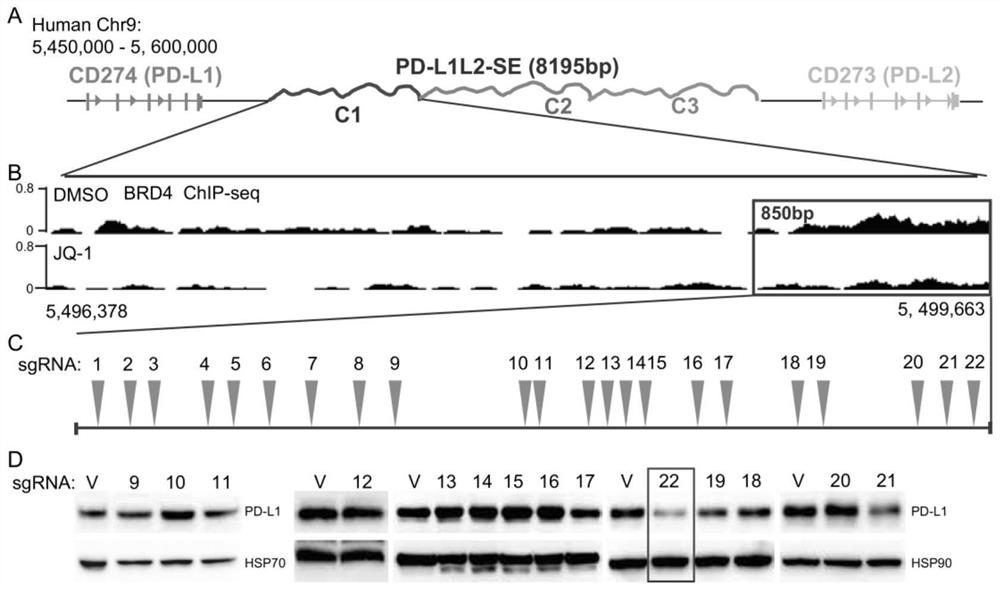
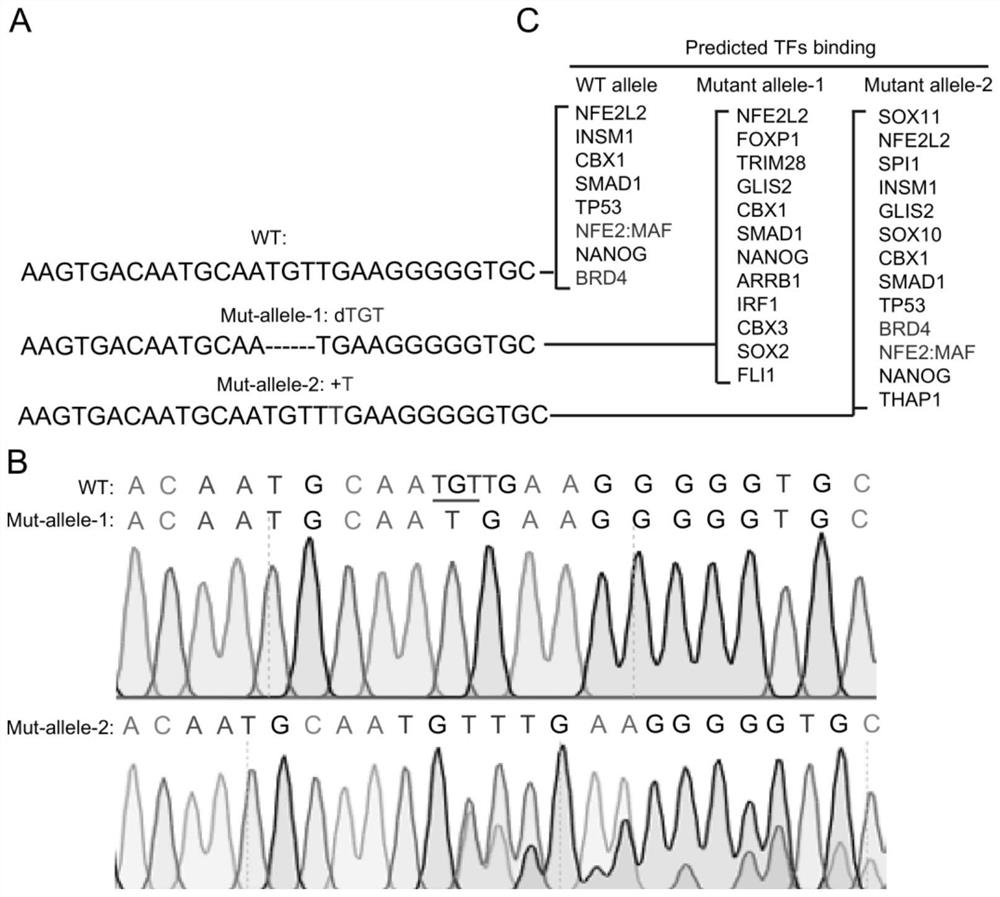
[0101] In order to fully understand the molecular mechanism of PD-L1L2-SE super-enhancer regulation of PD-L1 and PD-L2, the present invention carried out a fine analysis of PD-L1L2-SE, and found a core region (hg19: Chr9: 5,498, 950–5, 499, 663). In order to fully understand the function of this DNA sequence, the present invention designs all possible sgRNAs, 22 sgRNAs in total. By establishing stable sgRNA cell lines respectively, the present invention found that sgRNA-22 had the greatest effect on the expression of PD-L1 ( figure 1 shown in A-D).
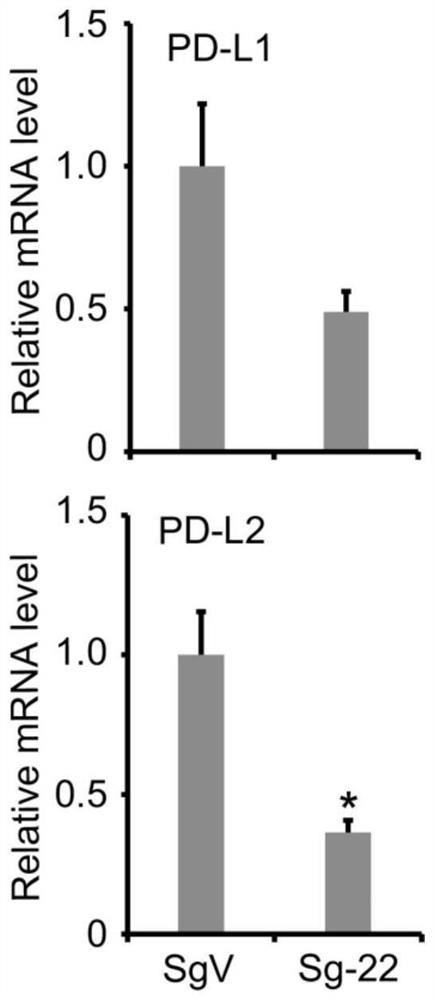
[0102] In order to further verify the result, the present invention further tested with quantitative RT-PCR, and found that the sgRNA-22 stable cell line can significantly down-regulate the expressions of PD-L1 and PD-L2 (such as figure 2 shown). The above results indicate that the DNA where sgRNA-22 is located is extremely important for the high expression of PD-L1 and PD-L2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com