Isosorbide mononitrate sustained release tablet and preparation method thereof
A technology of isosorbide dinitrate and sustained-release tablets, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and pill delivery, etc., and can solve the problems of uneven mixing of isosorbide mononitrate, stickiness, and high humidity requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
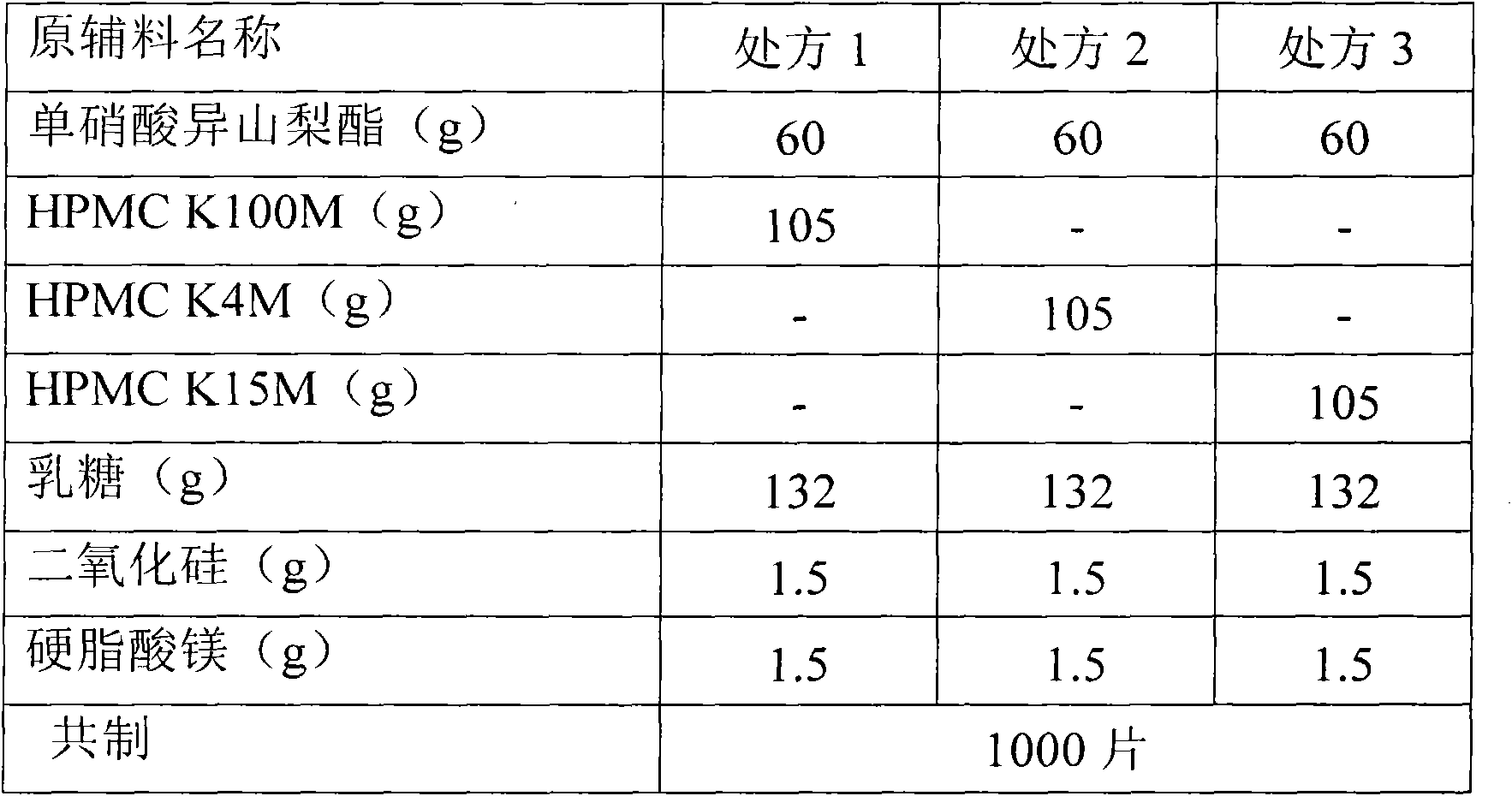
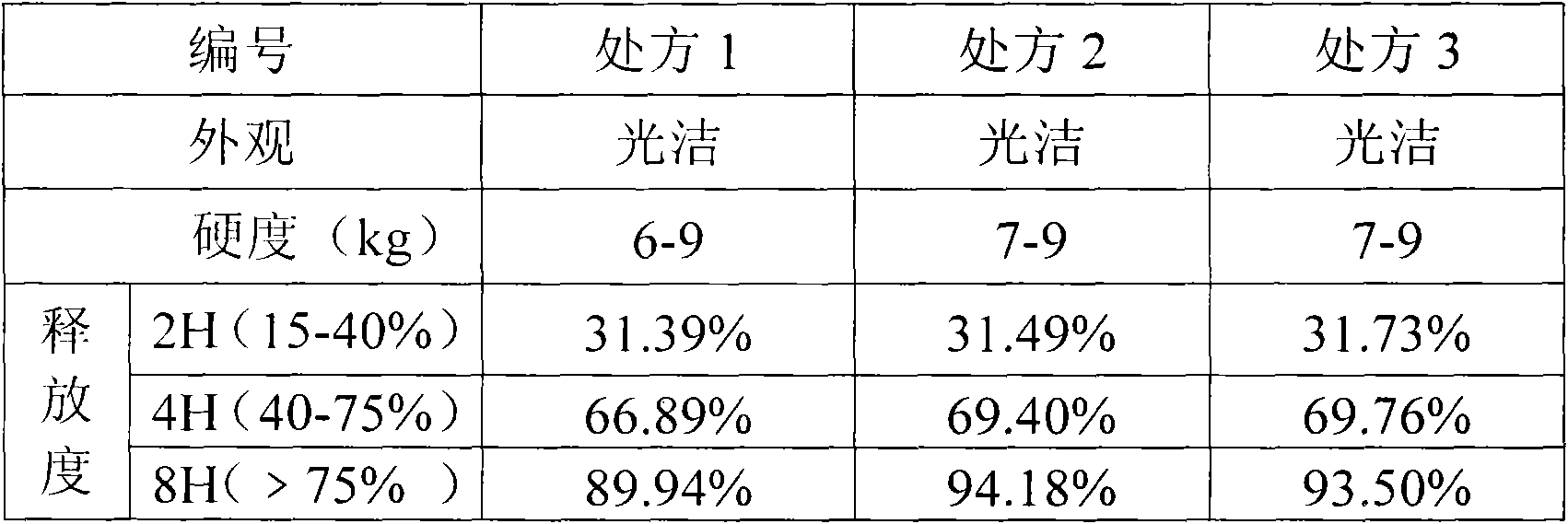
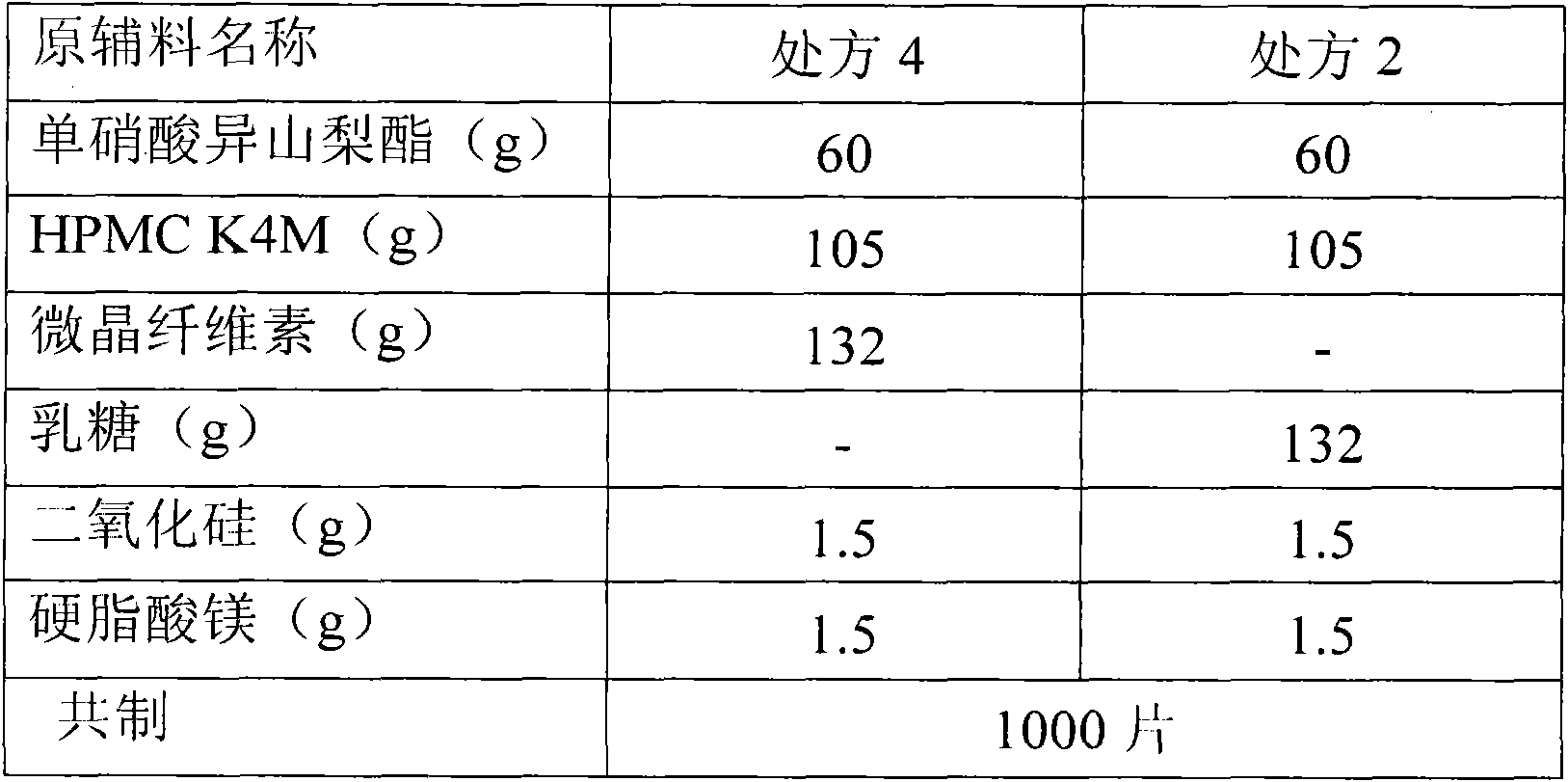
[0053] Isosorbide mononitrate 60g, hypromellose K4M 105g, microcrystalline cellulose 130.5g, silicon dioxide 3g, sodium lauryl sulfate 1g. A total of 1000 pieces were prepared.
[0054] 1) Isosorbide mononitrate is passed through a 30-mesh sieve and mixed with half of the prescription amount of hypromellose, then crushed through an 80-mesh sieve to obtain a mixed powder of isosorbide mononitrate and hypromellose, and the remaining hypromellose Pass through a 80-mesh sieve and set aside.
[0055] 2) Mix the mixed powder of isosorbide mononitrate and hypromellose with the remaining hypromellose and the prescribed amount of microcrystalline cellulose for 20 minutes, then add the prescribed amount of silicon dioxide and sodium lauryl sulfate and mix again 10 minutes.
[0056] 3) The powder is directly compressed into tablets to obtain plain tablets;
[0057] 4) Take half of the plain tablets for coating, take the prescribed amount of film coating premix Opadry II, slowly add it...
Embodiment 2
[0059] Isosorbide mononitrate 60g, hypromellose K4M 105g, microcrystalline cellulose 130.5g, silicon dioxide 3g, sodium lauryl sulfate 1.5g. A total of 1000 pieces were prepared.
[0060] Prepare with reference to the method of Example 1.
Embodiment 3
[0062] Isosorbide mononitrate 60g, hypromellose K4M 105g, microcrystalline cellulose 130.5g, silicon dioxide 3g, sodium lauryl sulfate 2g. A total of 1000 pieces were prepared.
[0063] Prepare with reference to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com