Method for treating herpes virus infection
A herpes virus infection and herpes virus technology, applied in antiviral agents, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve problems such as low solubility and difficult realization of diclofenac aqueous injection solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. Preparation of emulsion formula
[0033] The emulsion carrier ± [active ingredient] is prepared by the following steps:
[0034] 1. Preparation of the oil phase: Immerse the mixing vessel in a hot water bath (80±2°C). [Acyclovir, 50g] methylparaben (1g), propylparaben (1g), cetyl alcohol (60g), sorbitan monostearate 60 (12g), hard Fatty acid (steric acid) (20g), synthetic spermaceti (50g), dimethyl polysiloxane (30g), and triglyceride caprylic-capric acid ester (miglyol) 812 (70g) add this mixing vessel and stir to Mix well. The mixture was filtered once through a 150 mesh screen to remove particulates.
[0035] 2. Preparation of the aqueous phase: Immerse another mixing vessel in a hot water bath (80±2°C). [Diclofenac sodium salt, 50g] [Diclofenac lidocaine salt, 10g, 30g, or 50g] polysorbate 60 (36g), propylene glycol (160g), sodium citrate (10g), and sufficient to make the total weight reach 1000 g of pure water was added to the mixing vessel and s...
Embodiment 2
[0038] Example 2. Testing different compounds on animals
[0039] Target:
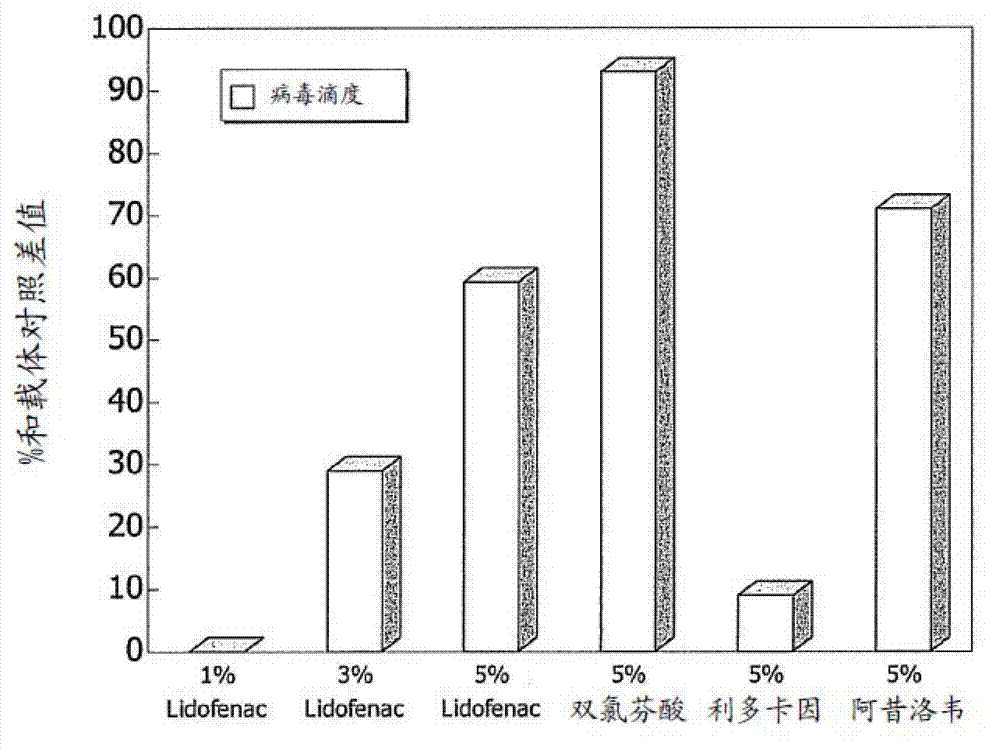
[0040] Test (1) ADO-1, ADO-2, ADO-3, and ADO-4 on herpes animal model; (2) 1% VDO99 emulsion, 3% VDO99 emulsion, 5% VDO99 emulsion and their placebo; ( 3) Effect of VGO99 emulsion (diclofenac sodium, 50 mg (RA009)), VDO99 emulsion (diclofenac, lidocaine salt 50 mg (RA052)), VAO99 emulsion (lidocaine, 50 mg (RA001)) and their placebo emulsions. The emulsion formulation ± active ingredient was prepared according to Example 1.
[0041] Test compound:
[0042] Group I
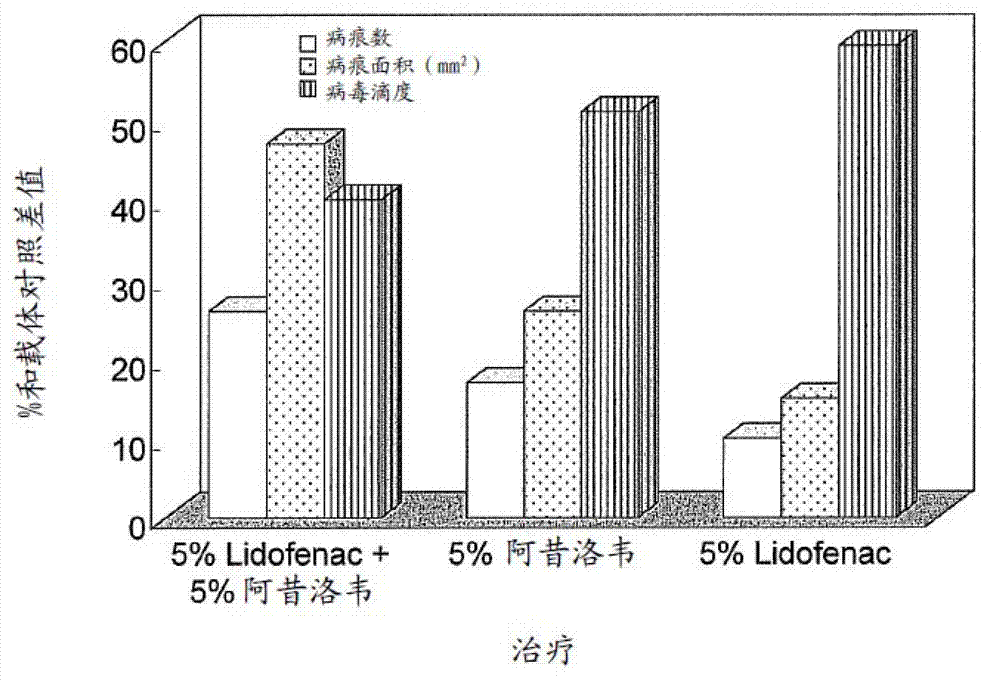
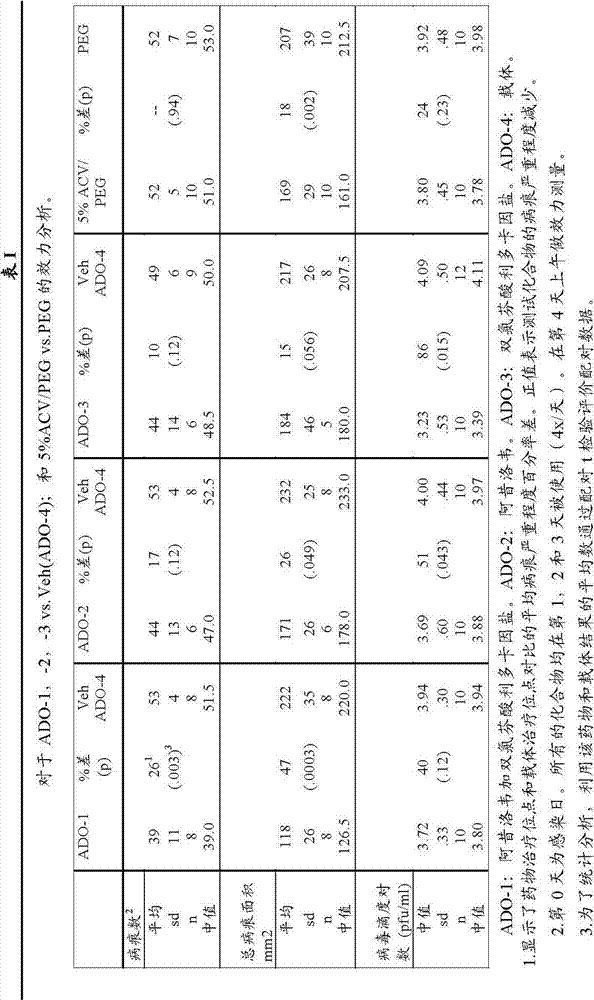
[0043] ADO-1: 5% acyclovir (50mg / g) plus 5% diclofenac lidocaine salt (Lidofenac50mg / g)
[0044] ADO-2: 5% acyclovir (50mg / g)
[0045] ADO-3: 5% diclofenac lidocaine salt (Lidofenac 50mg / g)
[0046] ADO-4: Vector.
[0047] Group II
[0048] 1% VDO99 emulsion: diclofenac, lidocaine salt (Lidofenac 10mg / g, RA032);
[0049] VDO99 placebo emulsion (RA035 placebo);
[0050] 3% VDO99 emulsion: diclofenac, lidocaine salt (Lidofenac 30mg / ...
Embodiment 3
[0122] Example 3. Clinical research protocol
[0123] Target:
[0124] To evaluate the effectiveness of VGO99 (5% diclofenac sodium emulsion) in patients with herpes zoster. The efficacy is judged by intensity ratings of pain and lesions.
[0125] study endpoint
[0126] Evaluate the following endpoints
[0127] · The primary efficacy endpoint was the time to complete cessation of shingles-related pain. Subjects were defined as achieving complete cessation of pain if they were pain-free for at least 7 days (pain reported as 0 on a numerical scale of 0-100).
[0128] Time to cessation of acute phase pain (pain experienced until all crusts fall off)
[0129] • A rough rate of subjects achieving complete cessation of shingles-related pain at the end of the follow-up period.
[0130] • Rough rate of subjects free of blisters, ulcers and crusts at the end of the treatment period and follow-up period.
[0131] • Percent reduction in VAS pain scores at specific clinical visits...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com