Anti-iga1 antibody
A technology of antibody and antibody fragment, which is applied in the direction of antibody, recombinant DNA technology, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. It can solve the problem of accelerating the disappearance of mouse antibodies and reducing the therapeutic effect of mouse antibodies, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0222] (1) Antigen preparation
[0223] An expression vector containing a cDNA encoding a full-length or partial-length IgA1 heavy chain is introduced into an enzyme that adds Gal to GalNAc bound to Ser / Thr on a polypeptide during synthesis of an O-linked sugar chain by the method described below. , proteins related to the activity of the enzyme or proteins related to the transport of UDP-galactose, etc. have been reduced or deleted in yeast, insect cells, animal cells, etc., thereby obtaining sugar chain-deficient IgA1 as an antigen protein or cells expressing sugar chain-deficient IgA1. In addition, a method of preparing an antigen by purifying sugar chain-deficient IgA1 from various human-derived cultured cells, human tissues, etc. expressing sugar chain-deficient IgA1 in large quantities on the cell membrane or in a culture medium, or preparing A synthetic peptide having a partial sequence of sugar chain-deficient IgA1 was used as an antigen. In addition, sugar chain-def...
Embodiment 1
[0360] Production of CHO Cell Line Highly Expressing Sugar Chain-deficient IgA1 on Cell Membrane
[0361] (1) Construction of membrane-type IgA expression plasmid pKAN932B8PVHmIgA
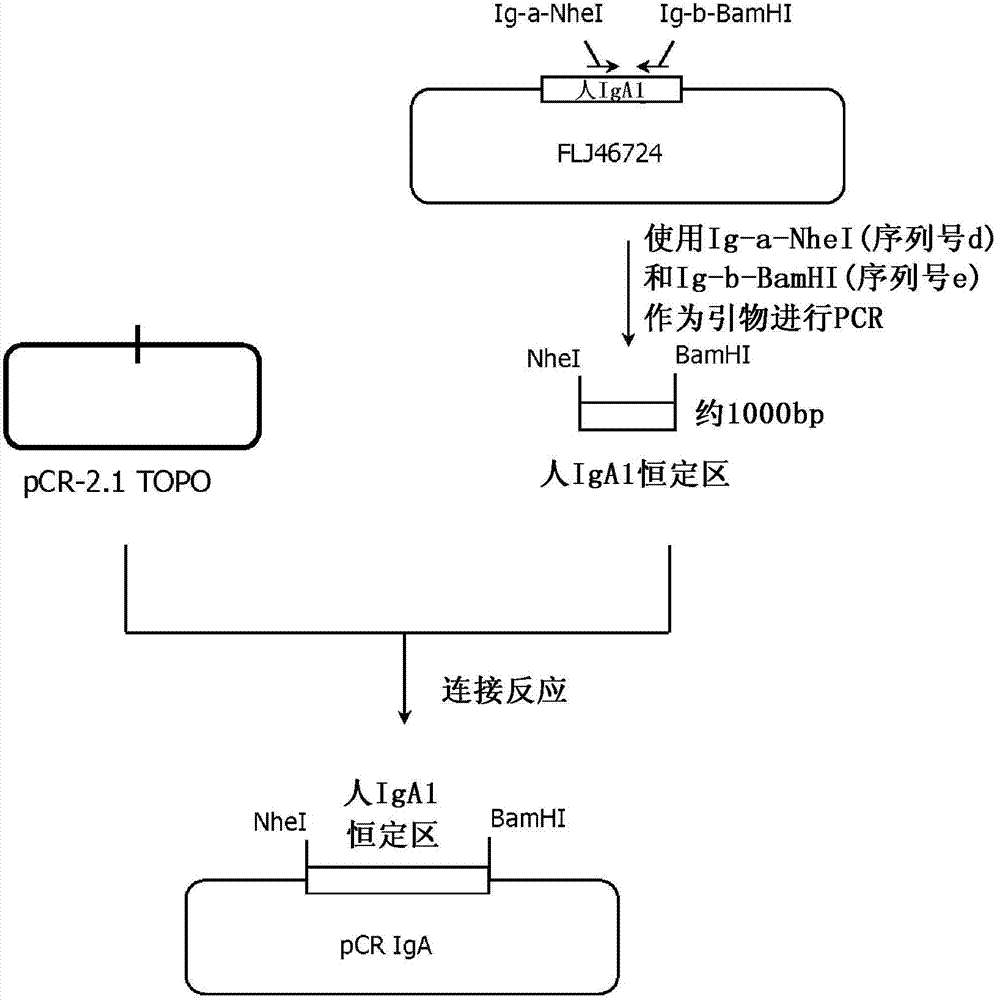
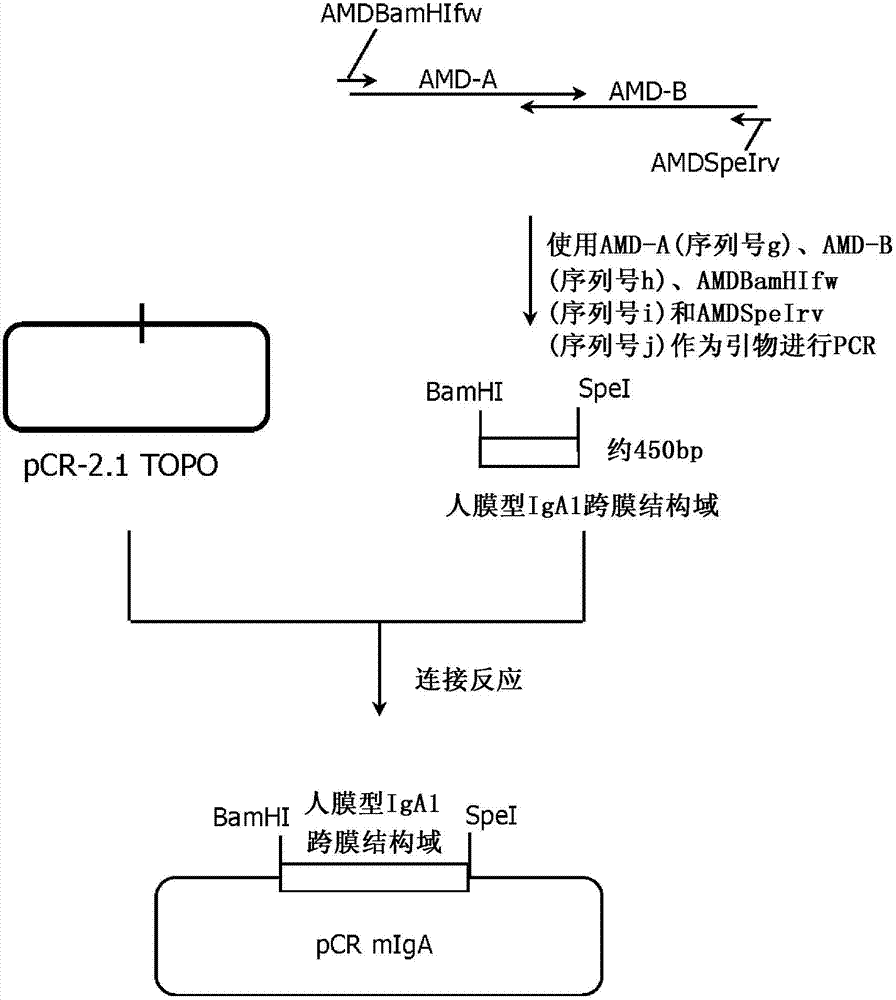
[0362] The vector pKAN932B8PVHmIgA for expressing membrane immunoglobulin A on the cell membrane was prepared in the following procedure. This plasmid is a plasmid vector for expressing a protein obtained by linking the heavy chain Fab portion of anti-CD20 antibody 2B8P to the constant region of membrane immunoglobulin described in Patent WO03 / 085107.
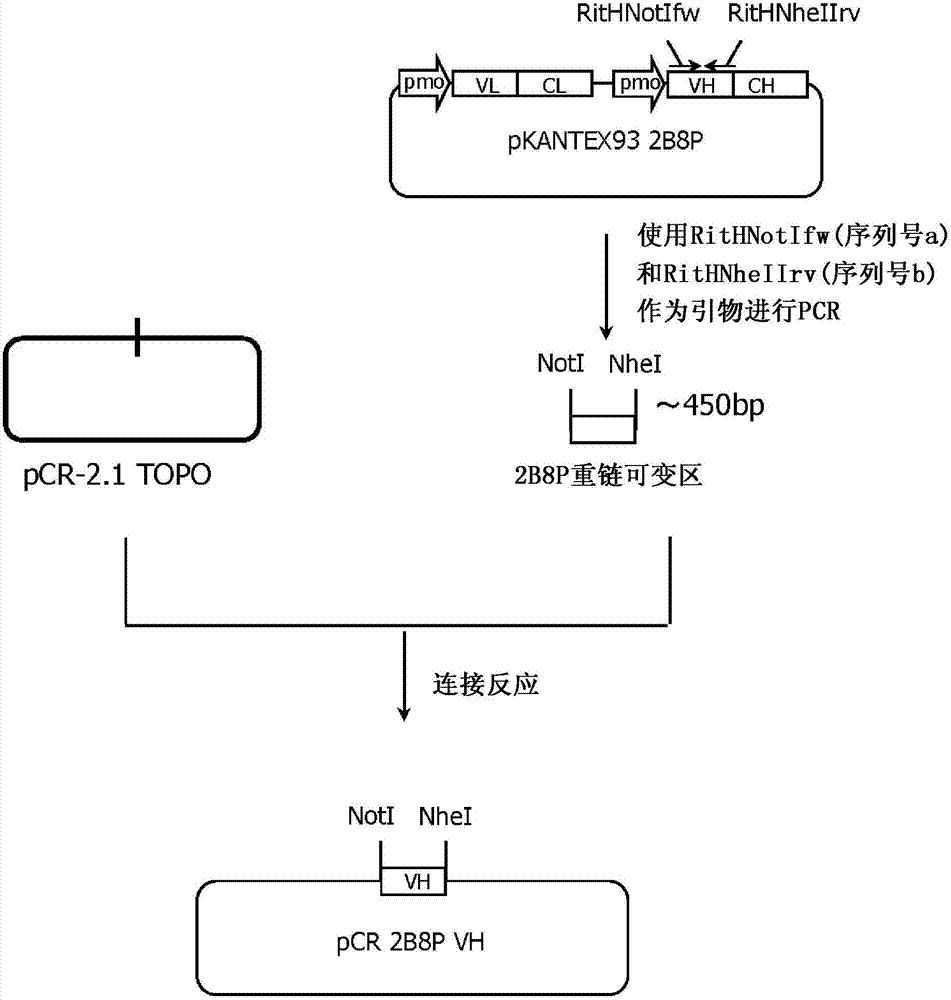
[0363] 1. Production of pCR2B8PVH
[0364] Using the plasmid vector pKANTEX932B8P described in Patent WO03 / 085107 as a template, the gene fragment including the heavy chain variable region of the anti-CD20 antibody 2B8P was amplified by the PCR reaction shown below. 1 ng of pKANTEX932B8P, 1 micromol / liter RitNotNheIfw (sequence number 4), 1 micromol / liter RitNotNheIrv (sequence number 5) and 2.5 units KOD polymerase (manufactured by Toy...
Embodiment 2
[0376] Preparation of sugar chain-deficient IgA1-Fc fusion protein
[0377] In order to obtain soluble mIgA1 protein, an Fc fusion protein mIgA1-Fc in which the extracellular region of mIgA1 is linked to human IgG4 Fc was designed. Specifically, a gene fragment in which a part of the extracellular region of mIgA1 was linked to human IgG4 Fc was prepared by the PCR method and inserted into pKAN932B8PVHmIgA obtained in Example 1 to prepare the Fc-fused mIgA1 expression vector pKANTEX-mIgA1- Fc. This expression vector was introduced into CHO / DG44 cell line and Lec8 cell line, and 500 μg / mL of G418 was added to the medium to screen the gene-transferred cells. The selected gene-introduced cells were cultured in serum-free medium Excell-302 (SAFC) for one week to obtain a culture supernatant containing mIgA1-Fc. Using a Mabselect (GE Healthcare) column, purification was performed from about 1 liter of the culture supernatant according to the attached instructions to obtain abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com