Oral magnesium hydroxide solid preparation
A magnesium hydroxide oral and solid preparation technology, applied in the digestive system, aluminum/calcium/magnesium active ingredients, powder delivery, etc., to achieve good effect, good taste and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Magnesium hydroxide 800g
[0046] Sodium Carboxymethyl Cellulose 200g
[0047] Orange Flavor 50g
[0048] Sucrose 950g
[0049] Makes 1000 bags.
[0050] Its preparation method is
[0051] 1) Pre-treatment: crush the magnesium hydroxide through a 100-mesh sieve, and separately crush the suspending agent, fragrance and flavoring agent through a 100-mesh sieve for later use;
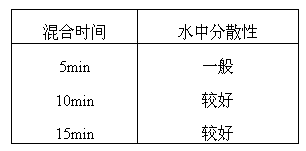
[0052] 2) Stirring and mixing: Weigh the above-mentioned raw and auxiliary materials in parts by weight according to the method of equal quantity addition, put them into a mixer and stir evenly, and the mixing time is 10 minutes;
[0053] 3) Sieve and mix: pass through a 100-mesh sieve and mix 3 times;
[0054] 4) Inspection-packaging: measure the particle content, check the appearance, sedimentation volume ratio of the dry suspension, and pack it after passing the inspection according to the quality standard requirements. Each bag contains 800mg of magnesium hydroxide.
Embodiment 2
[0056] Magnesium hydroxide 1200g
[0057] Sodium Carboxymethyl Cellulose 100g
[0058] Orange flavor 30g
[0059] Sucrose 670g
[0060] Makes 1000 bags.
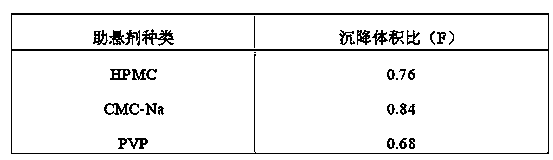
[0061] The preparation method is the same as in Example 1. According to the relevant requirements of the Chinese Pharmacopoeia 2010 edition on the sedimentation volume ratio inspection of dry suspensions: add 10ml of water to the magnesium hydroxide dry suspensions prepared in this example, the preparation has good dispersibility, shake vigorously for 1min, then let stand for 3h, Results The sedimentation volume ratio F=0.98, which meets the requirements of Pharmacopoeia.
Embodiment 3
[0063] Magnesium hydroxide 1600g
[0064] Sodium Carboxymethyl Cellulose 20g
[0065] Orange flavor 10g
[0066] Sucrose 370g
[0067] Makes 1000 bags.
[0068] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com