Intrauterine sustained control release drug delivery system adopting biodegradation material, and preparation method thereof
A biodegradable material and drug delivery system technology, applied in the field of biodegradable material intrauterine slow and controlled release drug delivery system and its preparation, can solve problems such as uterine injury, copper woman injury, and unreasonable shape of drug delivery system, and achieve recovery Fertility function, prevention of bleeding, effect of high-efficiency contraceptive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
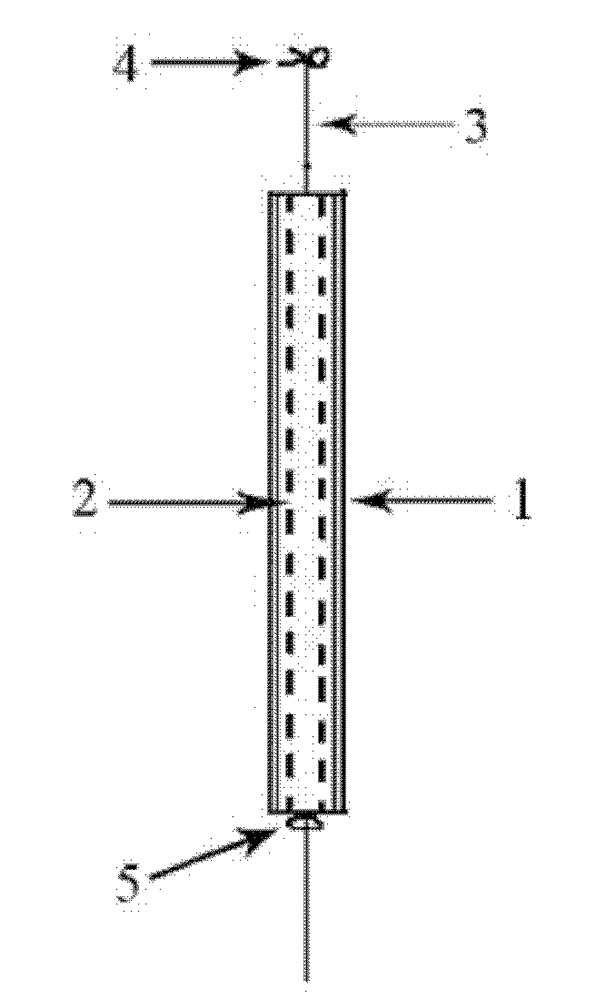
[0060] 1. Mix polycaprolactone and poloxamer (the particle size is 50 μ m, accounting for 3% of the total weight of the mixture), and extrude a pipe with an outer diameter of 3.0 mm and a wall thickness of 0.5 mm in a screw extruder to prepare a buffer Controlled release tubing, cut to 30mm. The extrusion temperature is 60°C, the screw speed is 15r / min, the traction speed is 20cm / min, the melt pump speed is 5r / min, the air flow rate is 7ml / min, and the molecular weight of polycaprolactone used is 2×10 4 g / mol.
[0061] 2. Set the molecular weight to 2×10 4 g / mol polycaprolactone and gestodene crystalline powder (mass ratio: 100:6) were stirred in a mixer at 70°C for 5 minutes to form a premix and then put into the mold of the molded rod, and the mold was placed in On a flat vulcanizer, preheat at 80°C for 2 minutes, then pressurize at 5Mpa for 1 minute, take it out, and cold press it for 2 minutes, and finally demould to obtain a gestodene-containing drug core with a diamete...
Embodiment 2
[0065] The difference from Example 1 is that the length of the drug core is changed to 30 mm, and the mass ratio of polycaprolactone to gestodene crystalline powder is 100:10, so that the effective length of the intrauterine drug delivery system is 24 mg / kg of gestodene. 30mm, the molecular weight of polycaprolactone used is 5.3×10 4 g / mol, the release amount is 30μg / d, and the service life is at least 2 years.
Embodiment 3
[0067] The difference from Example 1 is that the preparation method of the drug core containing gestodene is extrusion molding, and the molecular weight is 2×10 4 g / mol polycaprolactone and gestodene crystalline powder (mass ratio 100:6) were stirred in a mixer at 70°C for 5 minutes to form a premix, the screw speed was 20r / min, and the traction speed was 20cm / min, the melt pump speed is 5r / min, the air flow rate is 10ml / min, extrude a gestodene-containing drug core with a diameter of 1.8mm on the screw extruder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com