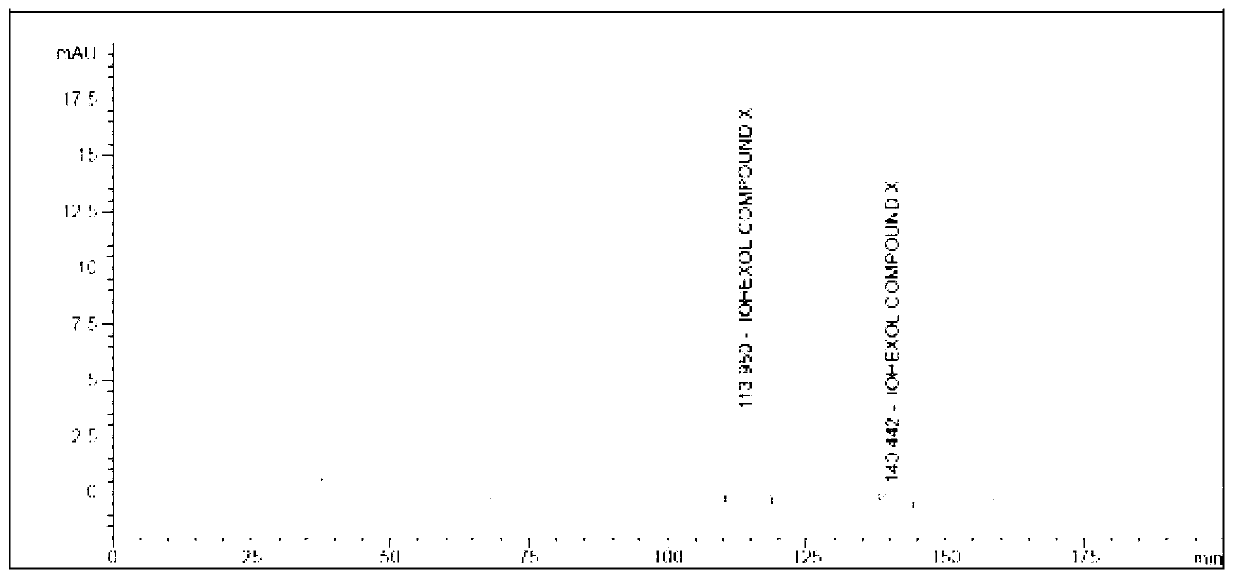
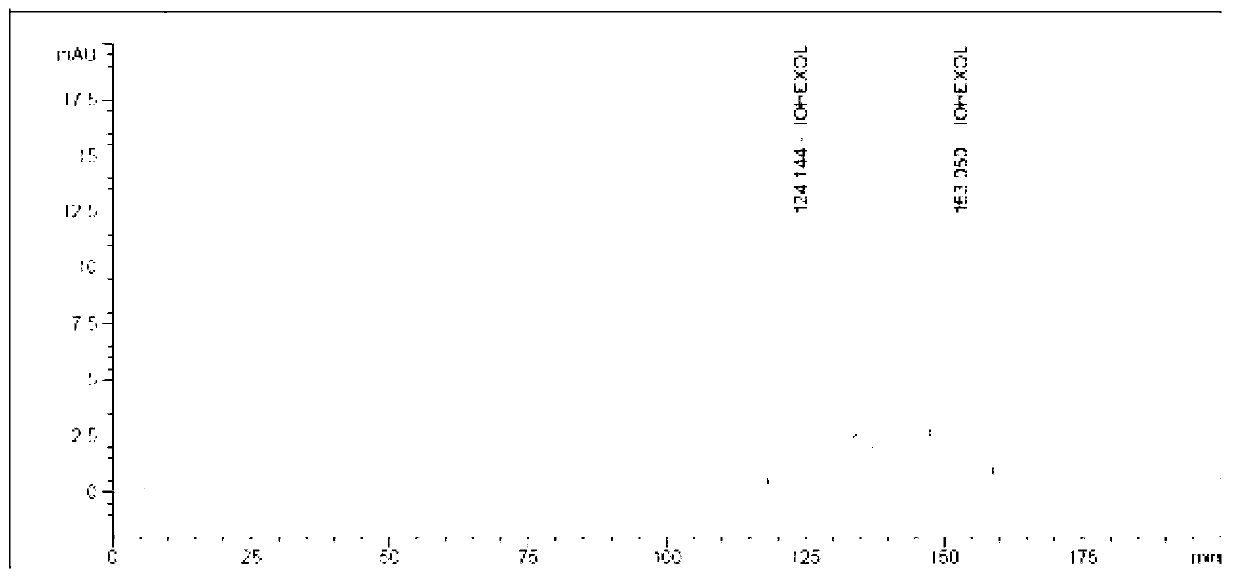
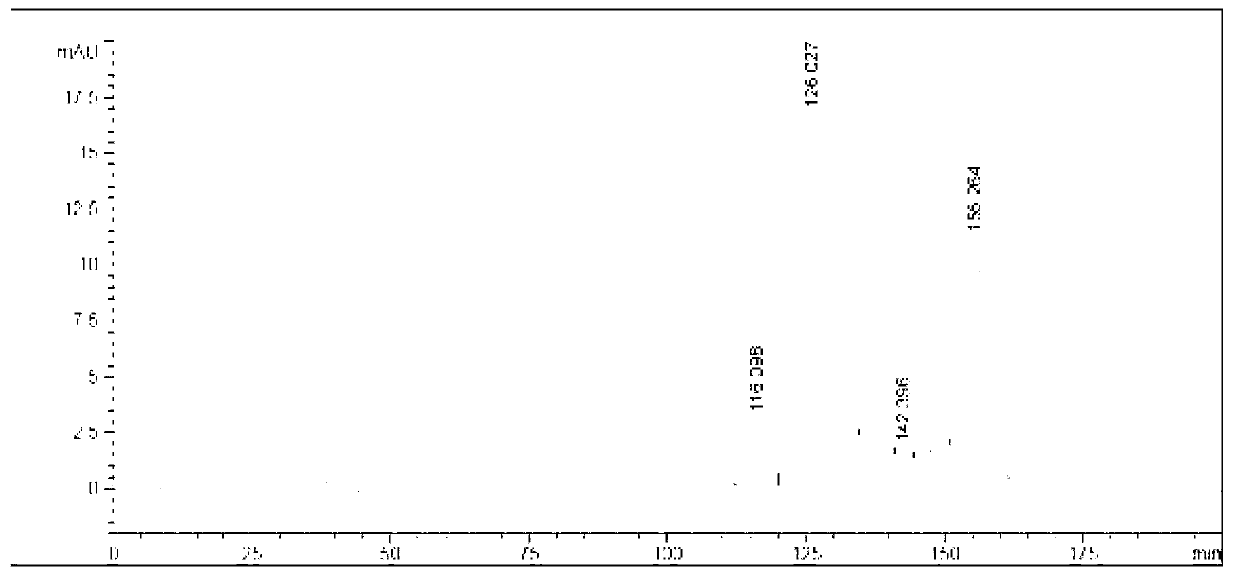
Preparation method of iohexol impurity
A technology of iohexol and propylene glycol, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of 3-acetylamino-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoic acid
[0048] Weigh the compound of formula (2) 3-amino-5-(2,3-dihydroxy-n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (40.0g, 63.3mmol) into a 500ml reaction flask , add acetic acid (200ml), acetic anhydride (34ml, 362.0mmol), concentrated sulfuric acid (0.5ml), heat up to 65°C, keep stirring for 24h, monitor by TLC until the reaction of raw materials is complete. The solid was filtered out, washed with water until the pH was neutral, and dried to give the compound of formula (3) as a white solid (43.0 g, 89.6%).
Embodiment 2
[0049] Example 2: Synthesis of 3-acetylamino-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoyl chloride
[0050] Add compound (3) 3-acetamido-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoic acid to a 500ml reaction flask equipped with a dry condenser (40.0g, 52.8mmol), chloroform (200ml), pyridine (2ml), stirred and heated to reflux at 70°C. A chloroform solution (200ml) of triphosgene (62.0g, 208.9mmol) was slowly added dropwise, and the addition was completed within 6h. After dripping, keep stirring until the solution is clear and keep warm for 3 hours, and TLC detects that the reaction is complete. Evaporated to dryness under reduced pressure to obtain formula (4) as a light yellow solid compound (38.4 g, 93.7%).
Embodiment 3
[0051] Example 3: 5-Acetamido-N-(2,3-diacetoxy-n-propyl)-N'-(1-hydroxymethyl-2-hydroxyethyl)-2,4,6-triiodo Synthesis of Isophthalamide
[0052] Add the above-mentioned compound of formula (4) (30.0g, 38.6mmol) into a 500ml reaction bottle, add chloroform (150ml), add 2-amino-1,3-propanediol (9.5g, 104.3mmol), stir at room temperature for 24h, TLC detection The chloroform phase was free of starting material and the reaction was terminated. Add water (300ml) and stir until the viscous matter dissolves, let stand to separate layers, separate the water phase and evaporate to dryness to obtain 39.0 g of yellow sticky solid compound of formula (5), HPLC purity 93%, directly proceed to the next step without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com