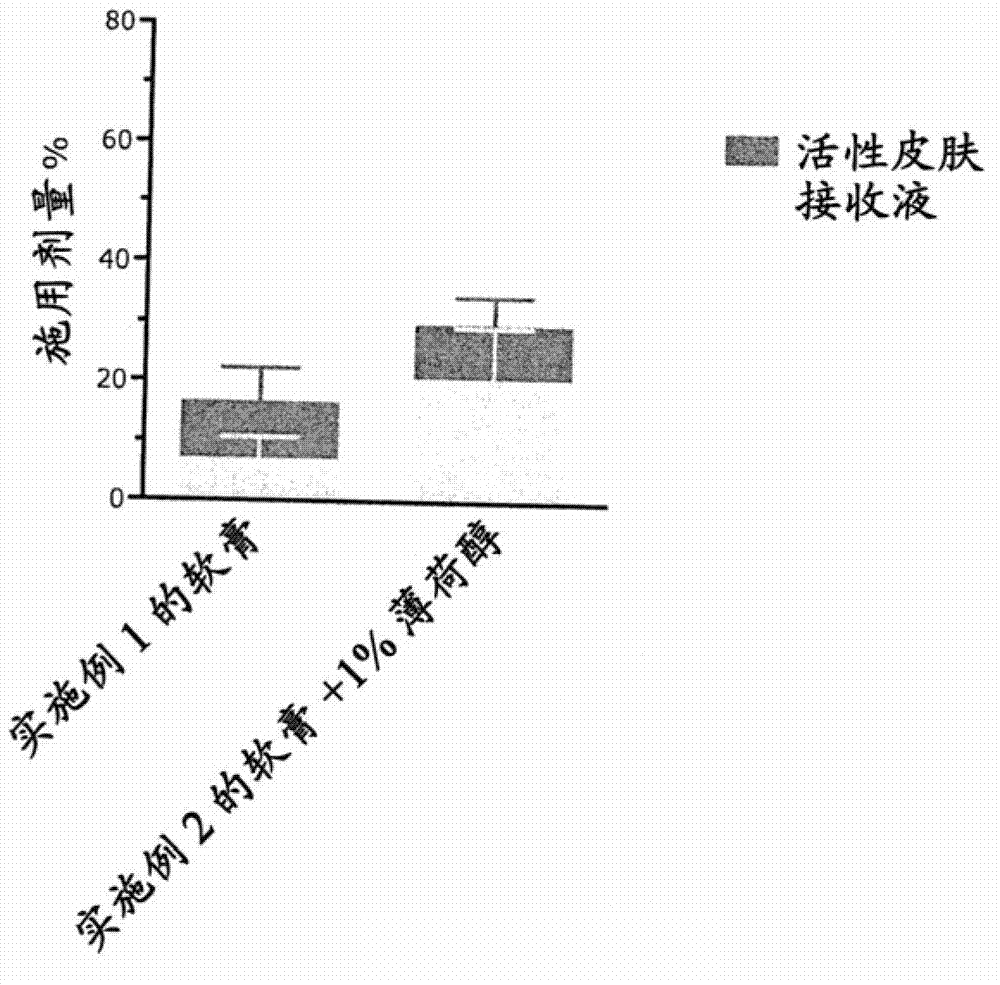
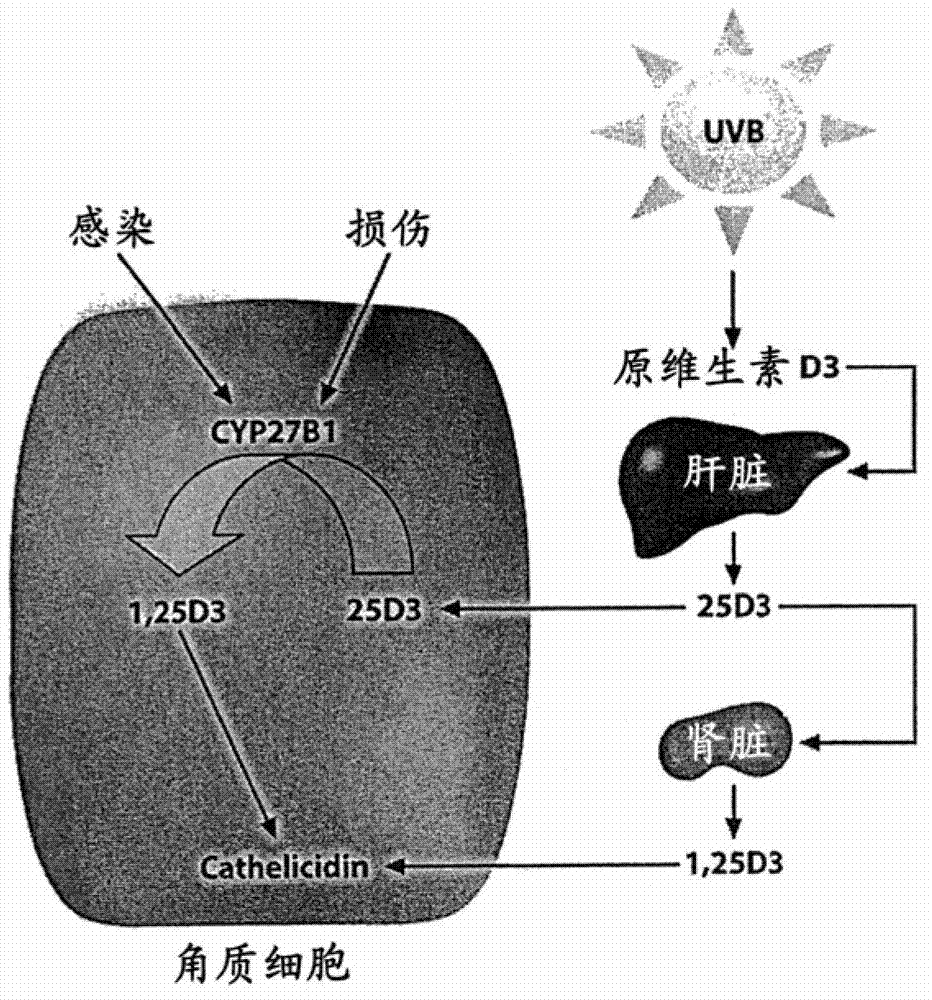
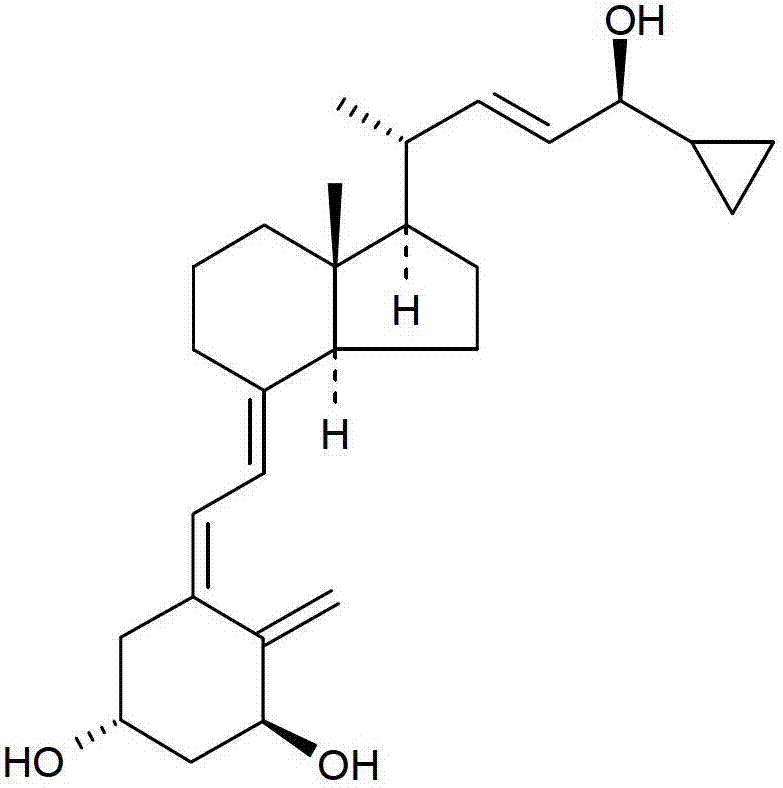
Pharmaceutical composition comprising solvent mixture and a vitamin D derivative or analogue
A composition and analog technology, which is applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve the problem of low ability to dissolve vitamin D analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0031] The vitamin D derivative or analog contained in the composition of the present invention may be selected from calcipotriol, calcitriol, tacalcitol, maxacalcitol, paricalcitol and alpha-calcitol . A preferred vitamin D analog that has proven effective in the treatment of psoriasis is calcipotriol. Calcipotriol may be in anhydrous or monohydrate form, preferably monohydrate, prior to dissolution in the solvent mixture.
[0032] In solvent mixtures used in the compositions of the present invention, the fatty acid ester solvent component is preferably selected from glycerides, such as fatty acids (e.g. C 6-24 triglycerides of fatty acids), and C 10-18Isopropyl esters of alkanoic or alkenoic acids such as isopropyl myristate, isopropyl palmitate, isopropyl isostearate, isopropyl linoleate, isopropyl monooleate, and octyl Lauryl alcohol or propylene glycol esters, such as propylene glycol dipelargonate. While medium chain triglycerides (as defined herein) are generally pr...
Embodiment 1
[0057] Test different solvent mixtures
[0058] Calcipotriol monohydrate was tested for solubility in compositions as shown in Table 1 below.
[0059] Table 1
[0060] Ingredient mg / g
[0061]Solubility was determined by shaking 3 ml of vehicle and excess calcipotriol monohydrate in a controlled room at 25±2°C for 24 hours. Experiments were performed as double assays. After 24 hours, the suspension was treated with -LCR filter for filtration, transfer the filtrate to a clean reaction tube and dilute with isopropanol. Concentrations were determined by reverse phase HPLC with UV detection (264 nm) compared to a standard curve.
[0062] The solubility of calcipotriol monohydrate in each solution is shown in Table 2 below.
[0063] Table 2
[0064] Solution 1
[0065] The results of this experiment indicated that the solubilization capacity of solution 6 containing medium chain triglycerides, N-methylpyrrolidone and polyoxypropylene-15-stearyl ether was ...
Embodiment 3
[0067] Stability of Calcipotriol in Different Solvent Mixtures
[0068] The compositions of the three solvent mixtures containing 25% NMP and 0% or 15% polyoxypropylene-15-stearyl ether are shown in Table 3 below.
[0069] table 3
[0070]
[0071] The solvent mixture was stored at 40°C for 1 month and at 25°C and 40°C for 3 months, after which the calcipotriol content was determined by HPLC. The results are presented in Table 4 below as a percentage of the initial assay.
[0072] Table 4
[0073] storage
[0074] The results showed that the only solvent mixture that showed satisfactory stability was the one containing 15% Solvent mixture 3 of E (polyoxypropylene-15-stearyl ether).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com