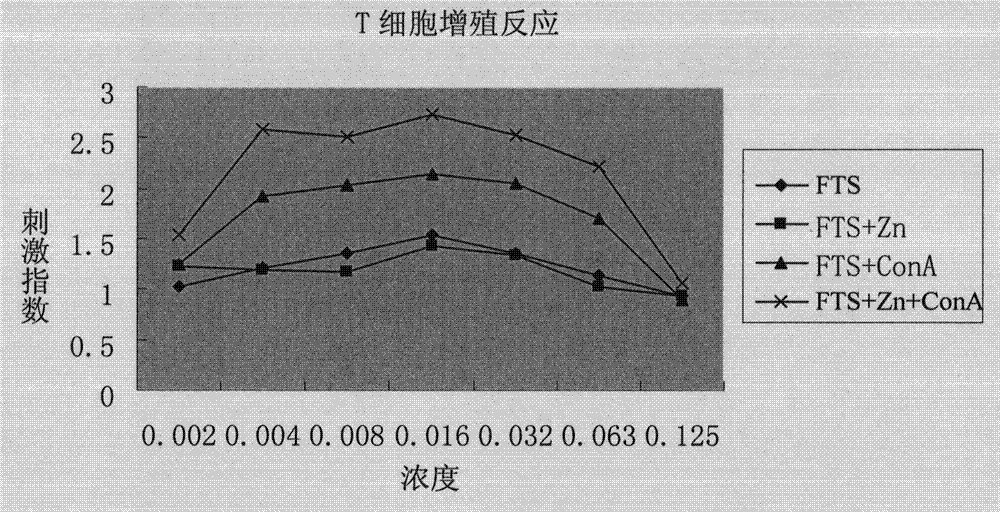
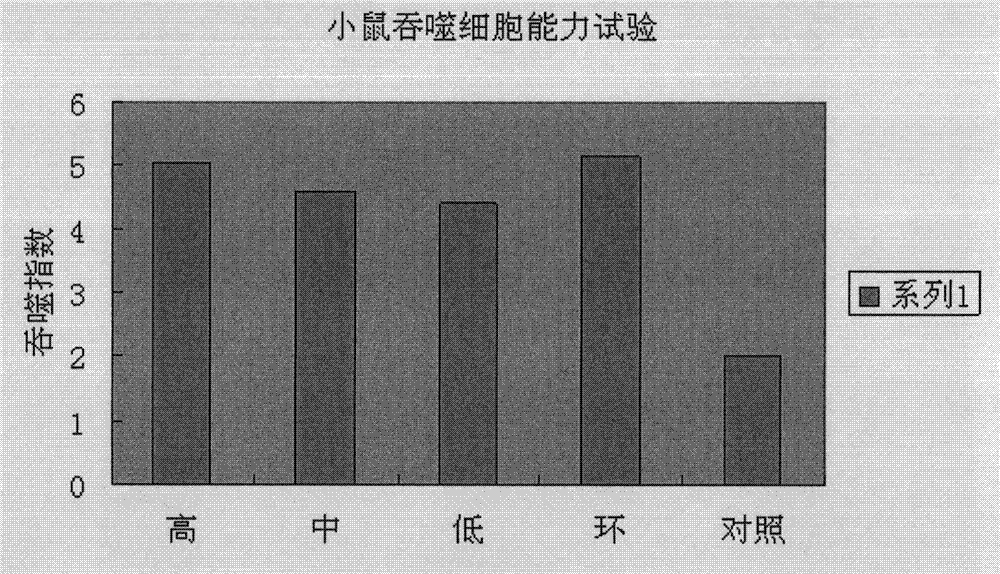
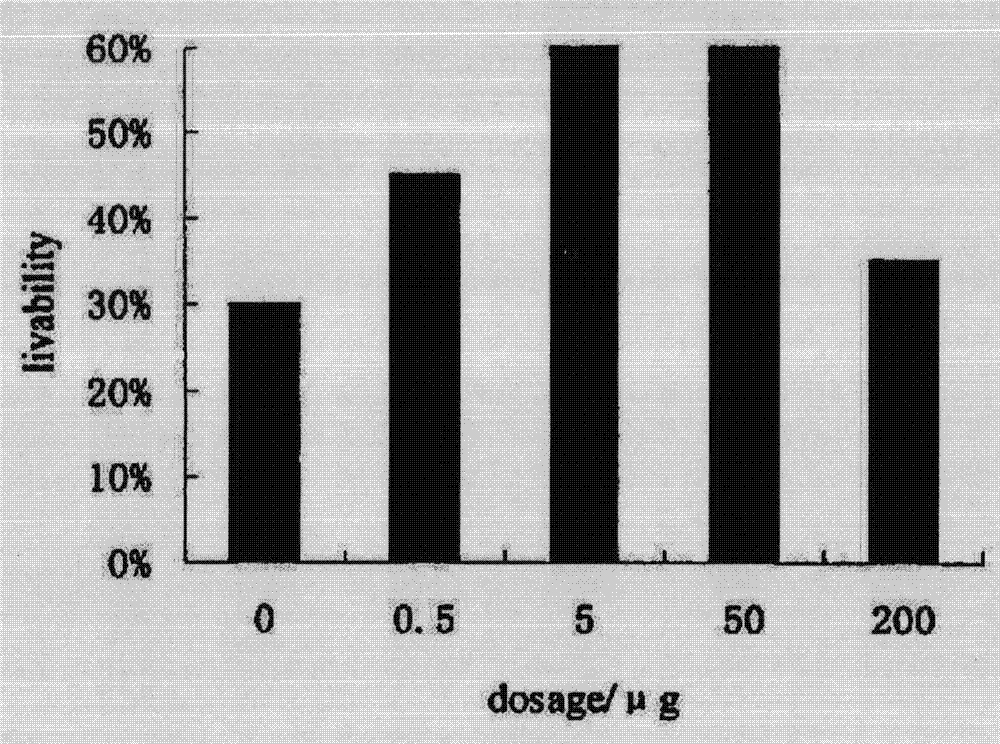
Application of serum thymus factor in preparation of protecting drugs of antineoplastic drugs, and tumour physical and chemical treatment drugs
A technology for serum thymus factor and drug, which is applied in the field of application of serum thymus factor in the preparation of antitumor drugs, protective drugs for tumor physics and chemotherapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This embodiment relates to the results of the EAC ascites tumor group
[0053] Administration by subcutaneous injection, animal inoculation on day 0, culture status checked on day 1, if no contamination, animals were weighed and randomly grouped to start administration. The tumor model mice were randomly divided into 5 groups, which were high-dose group, middle-dose group, low-dose group, positive control group and blank group, and the doses were 0.25 mg·kg -1 , medium dose 0.125mg·kg -1 , low dose 0.0625mg·kg -1 , except the positive drug group, administered once a day for 14 consecutive days; the positive drug group, 5-fluorouracil, once every other day, three times in total. The results are shown in Table 1:
[0054] Table 1 FTS is used for the result of anti-EAC ascites tumor drug ( n=10)
[0055]
[0056] △△ P△△△ P<0.001 compared with blank group; life extension rate compared with blank group.
[0057] The results in Table 1 show that the survival situat...
Embodiment 2
[0059] This example relates to the results of the H22 solid tumor group
[0060] Administration by subcutaneous injection, animal inoculation on day 0, culture status checked on day 1, if no contamination, animals were weighed and randomly grouped to start administration. The tumor model mice were randomly divided into 5 groups, which were high-dose group, middle-dose group, low-dose group, positive control group and blank group, and the doses were 0.25 mg·kg -1 , medium dose 0.125mg·kg -1 , low dose 0.0625mg·kg -1 , except the positive drug group, administered once a day for 14 consecutive days; cyclophosphamide administered once. The results are shown in Table 2.
[0061] Table 2 FTS is used for the result of anti-H22 solid tumor drug ( n=10)
[0062]
[0063] △△ P△△△ P<0.001 compared with blank group; tumor inhibition rate compared with blank group.
[0064] The results in Table 2 show that the tumor inhibition rate of the high-dose group and the middle-dose grou...
Embodiment 3
[0066] This embodiment relates to the results of the Walker-256 tumor group
[0067] Administration by subcutaneous injection, animal inoculation on day 0, culture status checked on day 1, if no contamination, animals were weighed and randomly grouped to start administration. The tumor model rats were randomly divided into 5 groups, which were high-dose group, middle-dose group, low-dose group, positive control group and blank group, and the dose was 2.2 mg·kg -1 , medium dose 1.1mg·kg -1 , low dose 0.55mg·kg -1 , except the positive drug group, administered once a day for 14 consecutive days; cyclophosphamide administered once. The results are shown in Table 3.
[0068] The next day after the last administration in the treatment group, the solid tumors were dissected out and weighed, and the tumor inhibition rate was calculated. The tumor inhibition rates of the administration groups were all greater than 30%, and there was a quantitative-effect relationship, and the cura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com