Method of manufacturing epsilon-caprolactam
A kind of caprolactam, crude technology, applied in the field of preparing ε-caprolactam, can solve problems such as high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
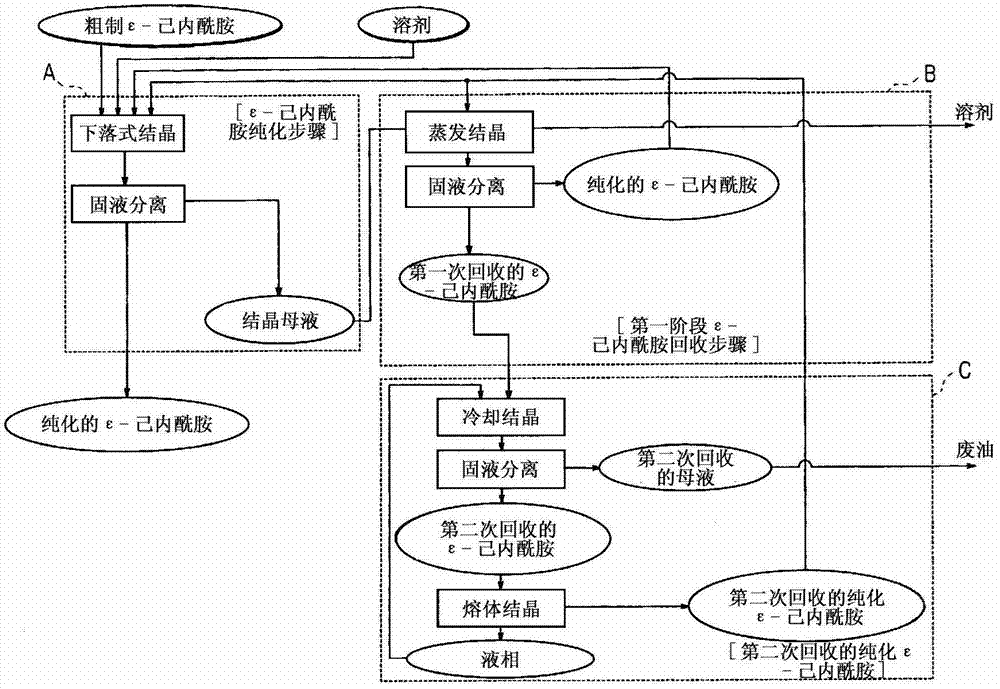
[0032] The preparation method of ε-caprolactam according to the present invention comprises ε-caprolactam purification step A, first stage ε-caprolactam recovery step B and second stage ε-caprolactam recovery step C. Each step will be described in detail below.
[0033] (ε-caprolactam purification step A)
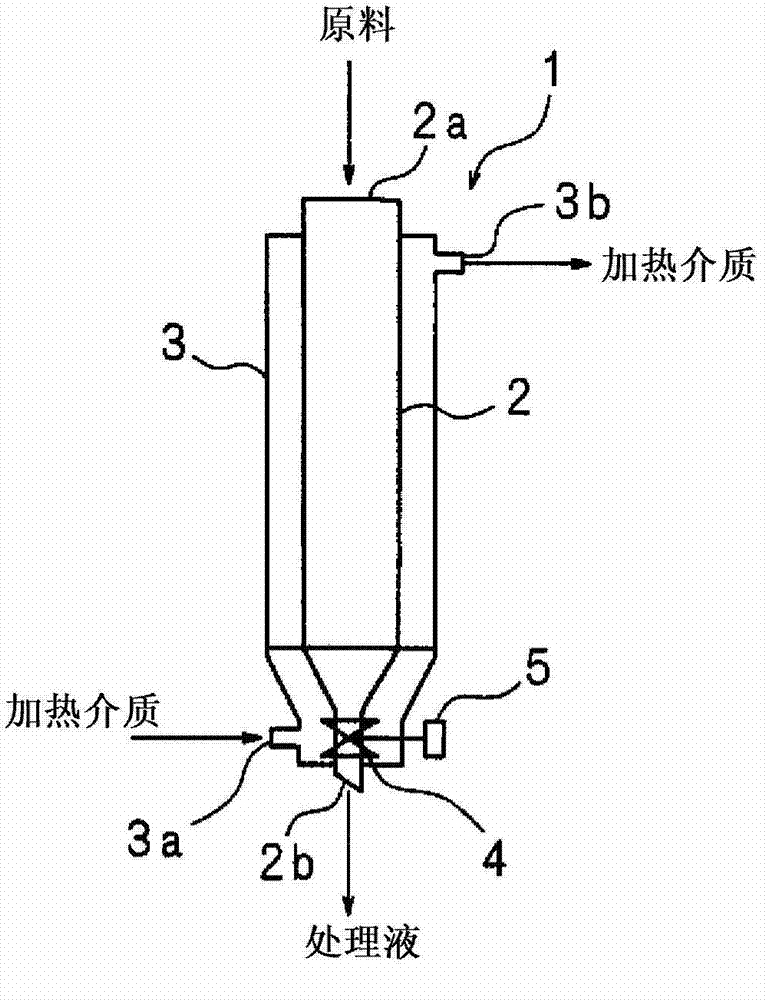
[0034]Crude ε-caprolactam was obtained by Beckmann gas-phase rearrangement of cyclohexanone oxime using a solid catalyst. Zeolite-based catalysts are suitably employed as solid catalysts. Crude ε-caprolactam in a heated molten state is poured into a crystallizer for drop crystallization [drop crystallization] together with a cooling solvent. As the solvent, examples thereof are straight-chain aliphatic hydrocarbons, side-chain aliphatic hydrocarbons, alicyclic hydrocarbons and the like having a carbon number of 6 to 12. Solvents that are insufficient solvents for ε-caprolactam such as n-heptane and cyclohexane are preferred. A crystallization temperature of about 40°C t...
Embodiment
[0052] Hereinafter, specific examples of the present invention will be described.
[0053] (ε-caprolactam purification step A)
[0054] Perform the following operations continuously. The flow rate is expressed in terms of weight per unit time. The reaction product was obtained by using a zeolite catalyst with a high silica content and causing a gas-phase Beckmann rearrangement reaction of cyclohexanone oxime under the temperature condition of 380° C. in the coexistence of methanol. Low melting point substances and high melting point substances were removed from the reaction product by distillation, whereby crude ε-caprolactam was obtained. The quality of crude ε-caprolactam obtained was based on GC (Gas Chromatography) measurements as follows: 99.131% ε-caprolactam; 139 ppm cyclohexanone oxime (OXM); 398 ppm 3-N-methyl-4 , 5,6,7-tetrahydrobenzimidazole (MTHI); and 430 ppm of 1,2,3,4,6,7,8,9-octahydrophenazine (OHP).
[0055] This crude ε-caprolactam was melted and its temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com