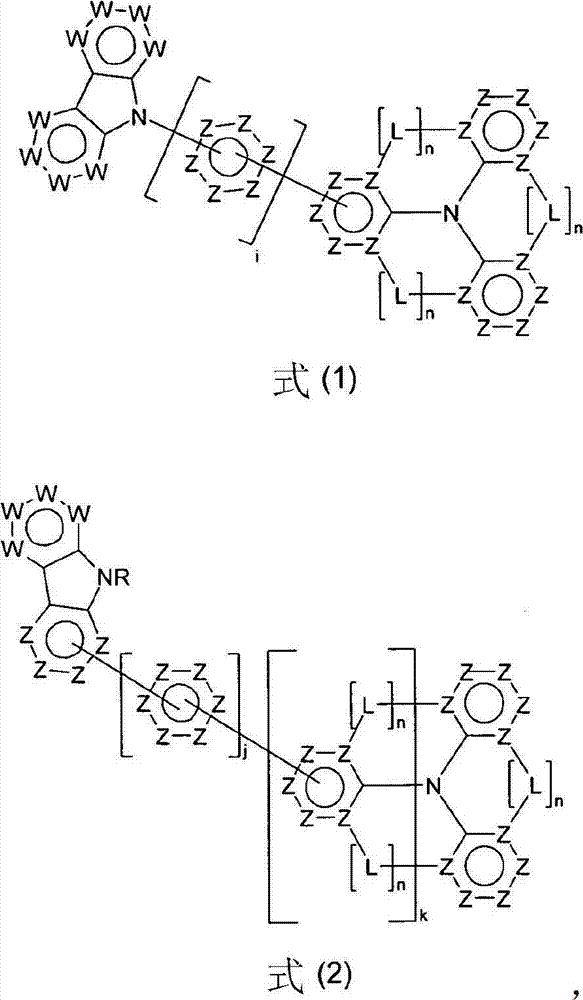
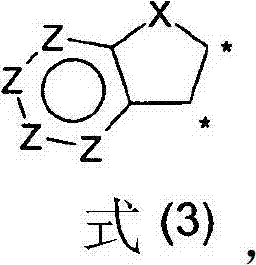
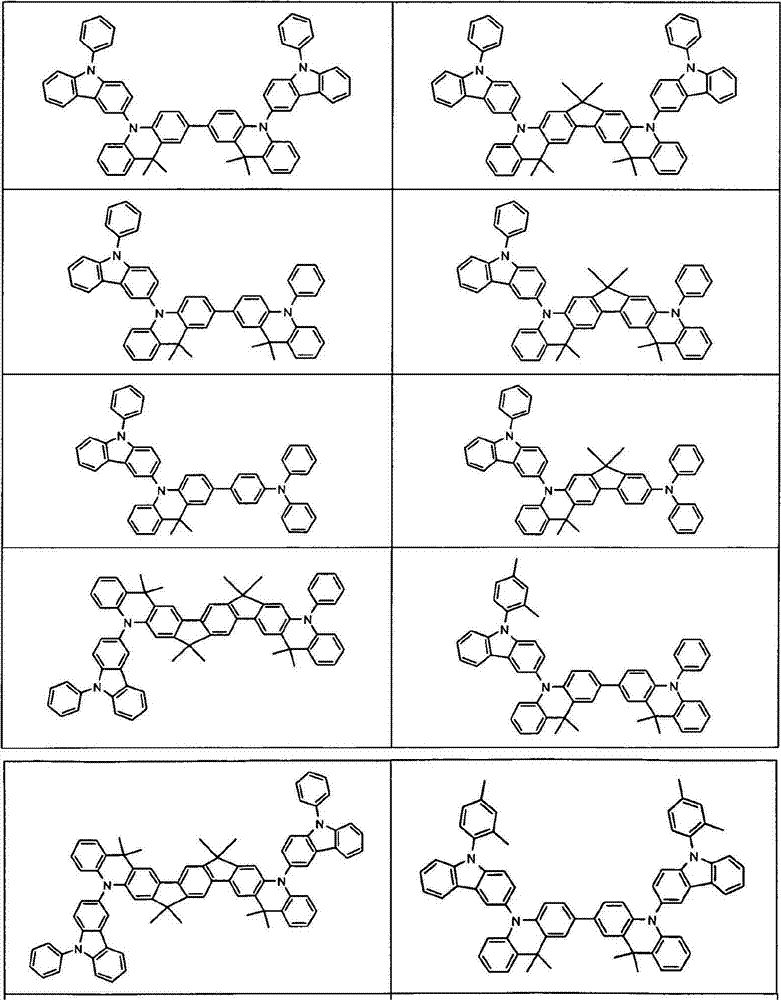
Compounds for electronic devices
A technology of compounds and atoms, applied in the field of electronic devices containing these compounds, can solve problems such as reducing the life of electronic devices and low electronic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0215] Example 1: 10-biphenyl-4-yl-9,9-dimethyl-2-(9-phenyl-9H-carbazol-3-yl)-9,10-dihydroacridine (HTM2) Synthesis
[0216]
[0217] a) Methyl 2-[4-(9-phenyl-9H-carbazol-3-yl)phenylamino]benzoate
[0218] Cesium carbonate (25.4 g, 78 mmol), palladium acetate (0.9 g, 4 mmol) and Xantphos (1.5 g, 8 mmol) were added to 3-(4-bromo-phenyl)-9-phenyl-9H-carbazole (CAS 1028647-93-9, 31.0 g, 78 mmol) and methyl anthranilate (10.1 ml, 78 mmol) in degassed toluene (300 ml), and the mixture was heated at reflux for 8 hours. The precipitated salts were filtered off and the mother liquor was evaporated under vacuum. The residue was extracted with chloroform in a Soxhlet extractor and subsequently recrystallized from toluene.
[0219] Yield: 26.0 g (55 mmol), 71% of theory, colorless solid.
[0220] b) 9,9-Dimethyl-2-(9-phenyl-9H-carbazol-3-yl)-9,10-dihydroacridine
[0221] Methyl 2-[4-(9-phenyl-9H-carbazol-3-yl)phenylamino]benzoate (26.0 g, 55 mmol) was added in portions to anhydro...
Embodiment 2
[0227] Example 2: Synthesis of 10-biphenyl-4-yl-2-(4-carbazol-9-ylphenyl)-9,9-dimethyl-9,10-dihydroacridine (HTM3)
[0228]
[0229] Similar to example 1, starting from 9-(4′-bromo-[1,1′-biphenyl]-4-yl)-9H-carbazole (CAS 212385-73-4) and methyl anthranilate , 10-biphenyl-4-yl-2-(4-carbazol-9-ylphenyl)-9,9-dimethyl-9,10-dihydroacridine was prepared in three steps.
[0230] Yield after sublimation: 7.2 g (12 mmol), 37% of theory, purity >99.9% by HPLC, colorless solid.
Embodiment 3
[0231] Example 3: Synthesis of 9,9-diphenyl-10-(9-phenyl-9H-carbazol-3-yl)-9,10-dihydroacridine (H3)
[0232]
[0233] Cesium carbonate (30.6 g, 94 mmol), palladium acetate (0.5 g, 2 mmol) and Xantphos (0.9 g, 5 mmol) were added to 9,10-dihydro-9,9-diphenylacridine (CAS 20474-15- 1, 15.7g, 47mmol) and 3-bromo-9-phenyl-9H-carbazole (CAS 1153-85-1, 15.1g, 47mmol) in a solution of degassed toluene (300ml), and the mixture Heated at reflux for 8 hours. The precipitated salts were filtered off and the mother liquor was evaporated under vacuum. The residue was extracted with toluene in a Soxhlet extractor. The crude product was subsequently recrystallized three times from toluene and sublimed twice in vacuum (p = 5 × 10 -5 mbar, T=280°C) for purification.
[0234] Yield: 10.5 g (18 mmol), 38% of theory, purity >99.9% by HPLC, colorless solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com