Medicinal use of 4-(4-phenylpiperazino)quinazoline derivatives
A technology of quinazolines and phenylpiperazines, applied in the field of biopharmaceuticals, can solve the problems of complex pathogenesis and unclear pathogenesis of schizophrenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
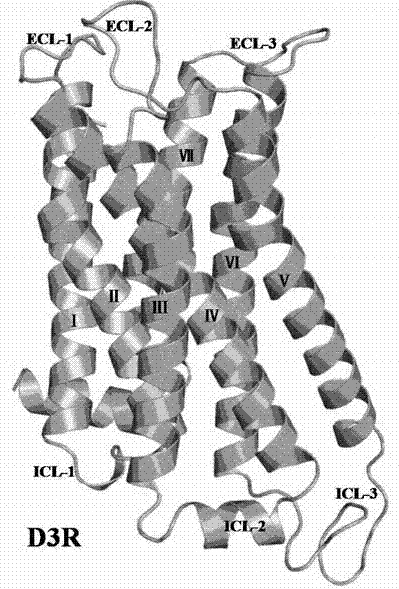
[0024] Example 1 Dopamine D 3 Receptor 3D structure optimization
[0025] Dopamine D 3 Receptors (Ellen Y. T. Chien, Wei Liu, Qiang Zhao, Vsevolod Katritch, Gye Won Han, Michael A. Hanson, Lei Shi, Amy Hauck Newman, Jonathan A. Javitch, Vadim Cherezov, Raymond C. Stevens. Science 330, (2010 ) 1091-1095. The PDB number is 3PBL) for energy optimization to exclude unreasonable structures in the receptor, thus obtaining the following figure 1 Optimized D shown 3 The three-dimensional structure of the receptor.
Embodiment 2
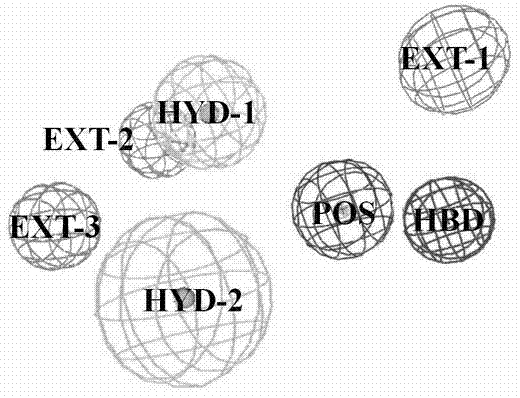
[0026] Example 2 Dopamine D 3 Active site detection of receptors and construction of pharmacophore models.
[0027] Dopamine D 3 The region within 10 ? of the conserved Asp3.32 in the receptor structure was defined as a box for site detection by the GRID program. Since the GPCR receptor pocket is negatively charged, four typical probes were selected: N+ (positive charge probe), O (hydrogen bond acceptor probe), N1 (hydrogen bond donor probe) and DRY (hydrophobic group probe). needles) to probe the corresponding electronegativity, hydrogen bond donor, hydrogen bond acceptor, and hydrophobic chemical environment in the protein, respectively. According to the binding energy between the GRID probe and the active pocket of the receptor and the nature of the interaction with the amino acid residues, an appropriate cluster is selected, and the geometric center and radius of rotation of the cluster are calculated.
[0028] The Catalyst module of Discovery Studio was used to c...
Embodiment 3
[0029] Example 3 Virtual screening and bioactivity testing based on receptor pharmacophore model
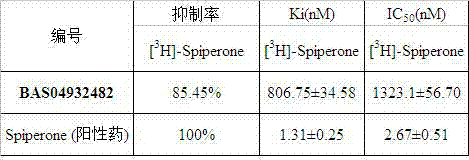
[0030]Use the best flexible method of the Catalyst module of the Discovery Studio program to search the MayBridge database (containing 61602 compounds), according to the matching value (Fit Value) of the small molecule and the pharmacophore, the docking of the small molecule and the receptor, the G-Score scoring function, and Small molecule ADMET property prediction results, 7 candidate compounds were selected from the ASINEX small molecule database (http: / / www.asinex.com), purchased from the Netherlands ASINEX company and tested for activity.
[0031] dopamine D 3 The receptor positive control substance was Spiperone, and both the test drug and the positive drug were dissolved in DMSO to 0.01 mol / L, and then diluted to 100 μmol / L with deionized water. Add 10 μL of the test compound and radioligand and 80 μL of the receptor protein into the reaction test tube, so that the fina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com