Method for preparing temsirolimus
A compound and organic solvent technology, applied in the field of preparation of temsirolimus, can solve the problems of highly toxic compounds, high price, and non-compliance with environmental protection and safety requirements, and achieve the effects of low cost, short preparation route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 2,2-bis(hydroxymethyl)propionic acid (5.00g, 37.28mmol) and triethylamine (26.41g, 0.26mol) into 50.00ml of N,N-dimethylformamide, ice-water bath, stir and add Tert-butyldimethylsilyl chloride (19.60 g, 0.13 mol) was raised to room temperature and reacted for 5-8 hours to obtain 10.0 g of compound Ⅰ-1.
[0030]
[0031] MS:363.22(M+H), 1 HNMR (CDCl 3 ): δ3.738(dd,4H);1.141(s,3H);0.914(s,18H);0.098(s,12H).
Embodiment 2
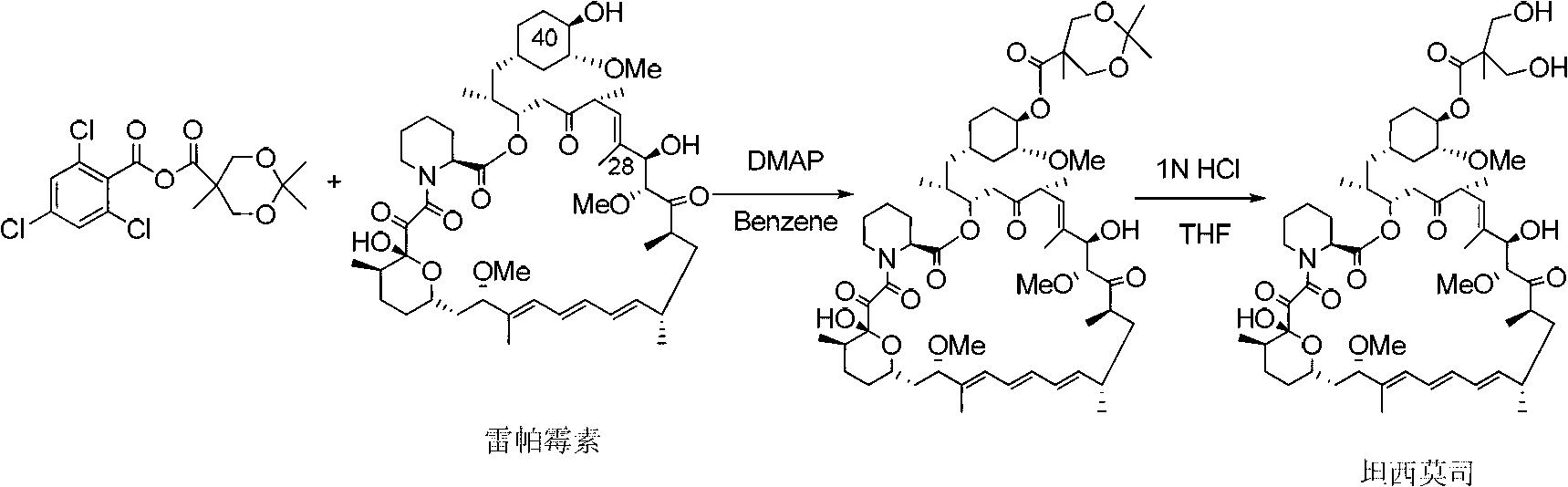
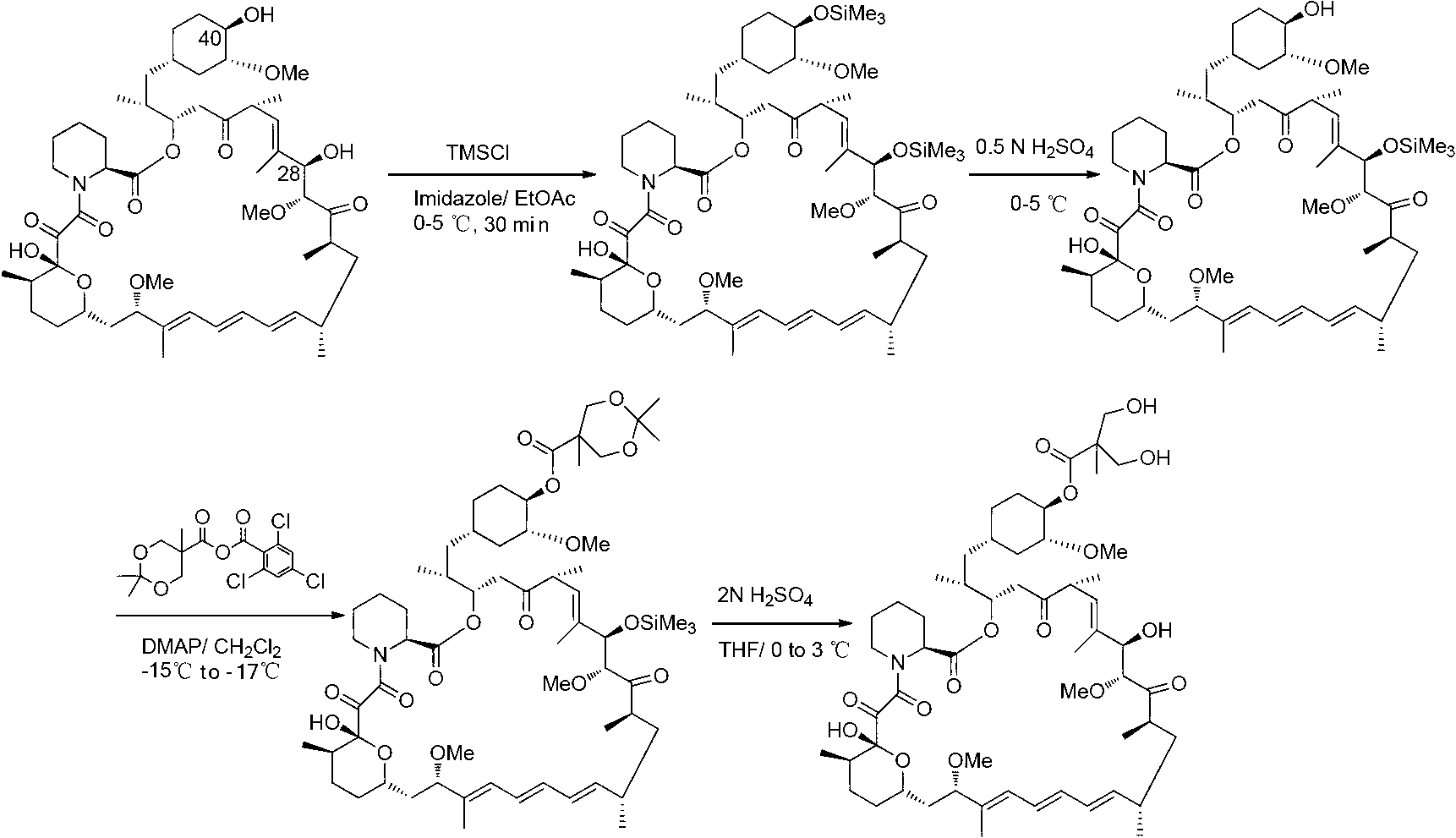
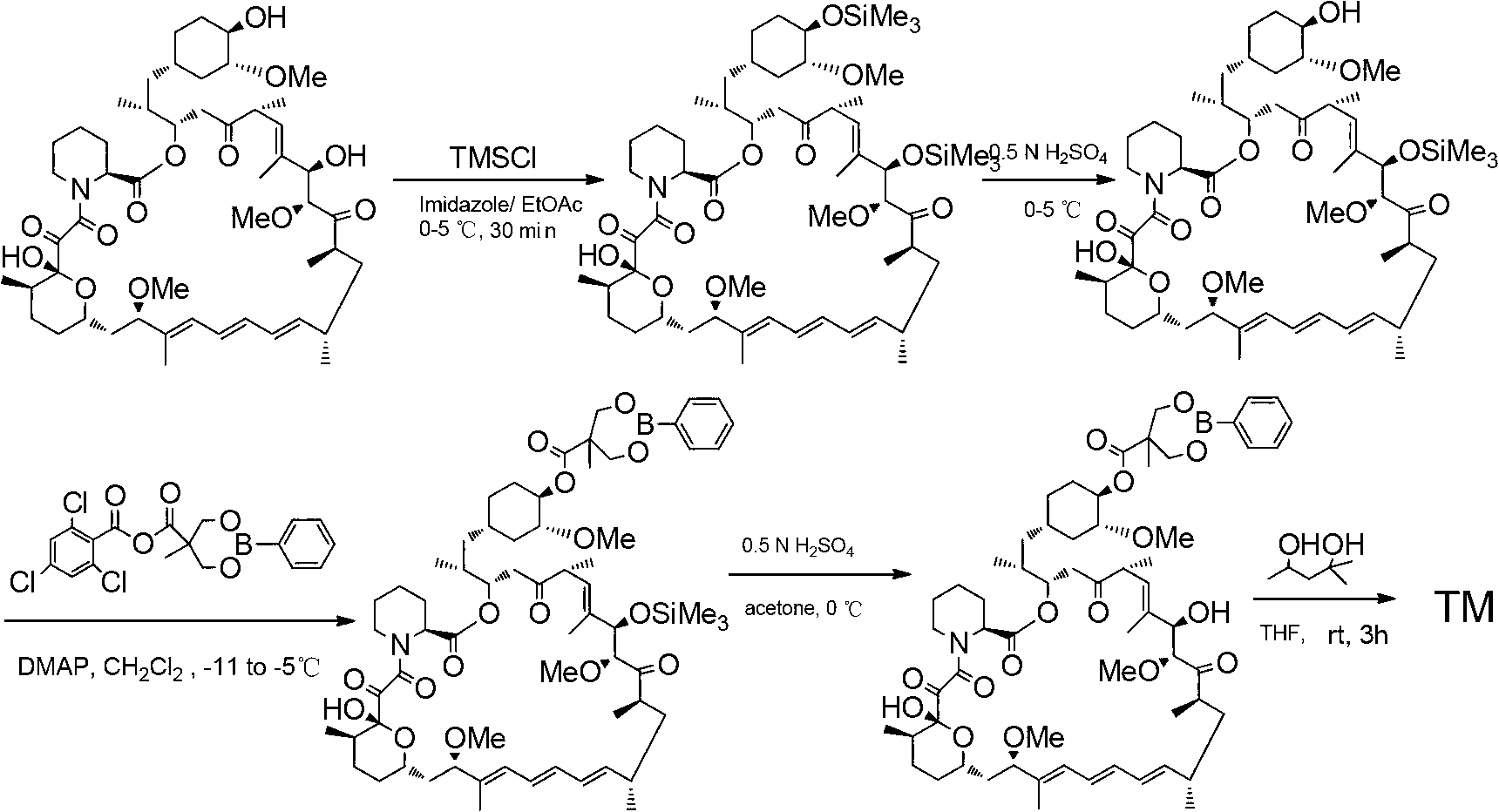
[0033] Add compound Ⅰ-1 (5.00g, 13.79mmol) and triethylamine (2.10g, 20.75mmol) into 50.00ml of dichloromethane, stir and add 2,4,6-trichlorobenzoyl chloride (3.30g, 13.53 mmol), after reacting at room temperature for 6 hours, slowly drop into 10.00ml dichloromethane containing rapamycin (4.20g, 4.60mmol) and 4-(N,N-dimethylamino)pyridine (2.25g, 18.38mmol) , and the reaction temperature was controlled at 0°C~5°C, and compound B-1 (3.47g) was obtained after 12 hours of reaction.
[0034]
[0035] MS:363.22(M+H), 1 HNMR (CDCl 3 ): δ4.65(m,1H,C(40)H);
[0036] 4.20((m,1H,C(28)H)4.17(d,2H);3.60(d,2H);1.06(s,3H);0.87(s,18H);0.034(s,12H).
Embodiment 4
[0038] Add compound B-1 (1.00g, 0.79mmol) into a solvent containing 15.00ml of acetone, stir and add 1N hydrochloric acid (2.38ml) in ice-water bath for 1~2h to prepare Tesirolimus (0.75g).
[0039] MS:1052.89(M+23), 1 HNMR was identical to the product described in Example 11 of US5362718.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com