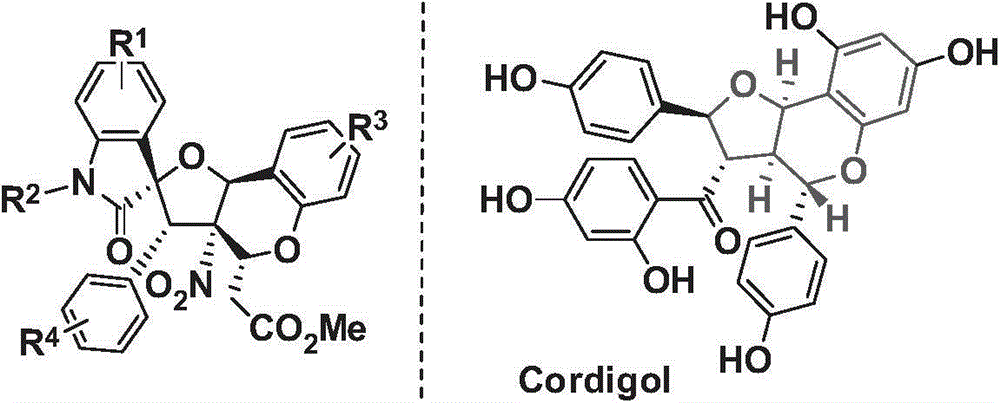
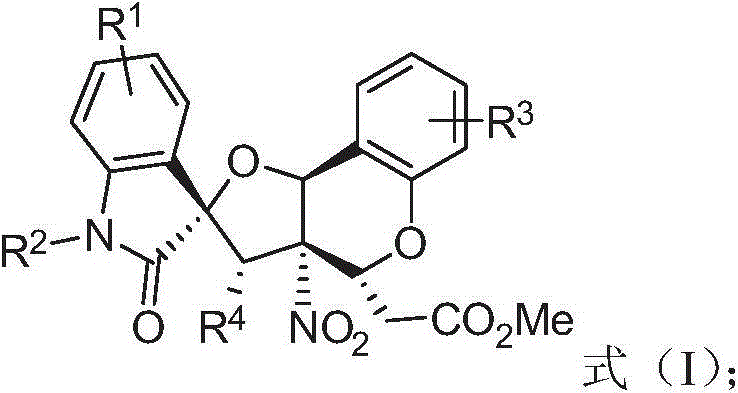
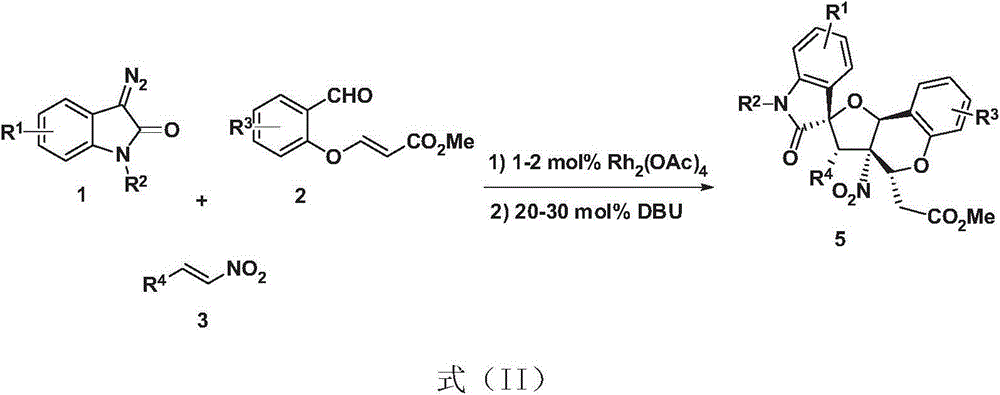
Tetrahydrofuran benzodihydropyran polycyclic compound and application thereof
A technology of chroman and polycyclic compounds, which is applied in the field of pharmaceutical synthesis chemistry, can solve the problems of poor chemical selectivity and long reaction time, and achieve high yield, short preparation route and low synthesis cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 prepares compound 5a of the present invention
[0025] Weigh trans-phenylnitroalkene 3a (0.20mmol), rhodium acetate (1.70mg, 0.004mmol), 5-bromo-ortho-substituted benzaldehyde 2a (0.30mmol), Molecular sieves (70 mg) were put into a small test tube reactor, and 1.0 mL of redistilled dichloromethane was added at room temperature. N-Methylisatin diazo 1a (0.30mmol) was dissolved in 0.7mL redistilled dichloromethane, and injected into the reaction system through a peristaltic pump for 1 hour. After the injection was completed, DBU (0.04mmol) was added, and the reaction was continued for 2h , the reaction was completed, and the solvent was removed by rotary evaporation at 40°C, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:50 ~ 1:20) to obtain tetrahydrofuranochroman compound 5a. Yield 56%, dr 82:18. See Table 1.
[0026]
[0027] Characterization of the product tetrahydrofuranochroman compound 5a:
[0028] 1 H ...
Embodiment 2-19
[0030] Embodiment 2-19 prepares compound (5b~5s)
[0031] Embodiment 2-19 is the same as embodiment 1. See Table 1 for the changes of substituents, compound number, d.r. value, yield, etc. in the reaction.
[0032] Table 1
[0033]
[0034] The characterization of the product tetrahydrofuranochroman compound 5b-5s is as follows:
[0035] Characterization of 5b:
[0036] 1 H NMR (400MHz, CDCl 3 , 25℃, TMS): δ=8.03(d, J=8.1Hz, 1H), 7.72(d, J=2.2Hz, 1H), 7.55-7.17(m, 8H), 6.91(dd, J=11.0, 8.2Hz, 4H), 4.94-4.86(m, 1H), 3.83(d, J=2.5Hz, 4H), 3.28-3.08(m, 1H), 2.94(dd, J=17.6, 8.4Hz, 1H), 2.30(s, 3H);
[0037] 13 C NMR (400MHz, CDCl 3 ,25℃,TMS):δ=175.94,171.23,169.56,153.29,140.15,133.52,131.45,130.07,129.49,129.17,128.99,126.38,125.51,123.63,120.41,119.01,116.68,115.17,93.11,86.20,76.63 , 75.99, 62.89, 54.29, 47.64, 34.93, 26.32.
[0038] Characterization of 5c:
[0039] 1 HNMR (400MHz, CDCl 3 , 25℃, TMS): δ=7.93(d, J=7.0Hz, 1H), 7.43(d, J=8.7Hz, 1H), 7.39-6.96(m, ...
Embodiment 20 4
[0089] Example 20 Inhibition of Histone Deacetylase Activity by Tetrahydrofuranochromanane Polycyclic Compounds 5a-5s
[0090] Histone deacetylase (HDAC) is a class of proteases that play an important role in the structural modification of chromosomes and the regulation of gene expression. In cancer cells, overexpression of HDAC leads to enhanced deacetylation, which increases the attractive force between DNA and histones by restoring the positive charge of histones, making the relaxed nucleosomes very compact, which is not conducive to specific genes expression of some tumor suppressor genes. Histone deacetylase inhibitors (HDACi) can regulate the expression and stability of apoptosis and differentiation-related proteins by increasing histone acetylation in specific regions of chromatin, and induce apoptosis and differentiation. Become a new class of anticancer drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com