Methanol Steam Reforming Catalyst
A catalyst and steam technology, applied in the field of methanol steam reforming catalyst, can solve the problem that the catalyst is not easy to be used in large-scale commercial use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: PtV-Zr / CeO 2
[0052] A first solution containing Pt and V was prepared by dissolving 15.244 g of hexahydroxy(IV) platinum acid (H 2 Pt(OH) 6 ) solution (16.40% by weight Pt), 1.795g NaVO 3 and 1.484g Zr(OH) 4 Add 35g of water and stir.
[0053] By mixing 7.5g urea and 0.461g Na 2 CO 3 A second solution was prepared by adding 30.0 g of water. The second solution was then mixed with 50.0 g of CeO in a ball mill bottle 2 (HSA20 from Rhodia), resulting in the formation of a dense slurry. The first solution is then contacted with a dense slurry. The resulting mixture was ball milled until about 90% of the solid particles were less than about 10 μm in diameter. Subsequently, the slurry was dried at about 120°C for about 2 h and calcined in air at about 550°C for 4 h.
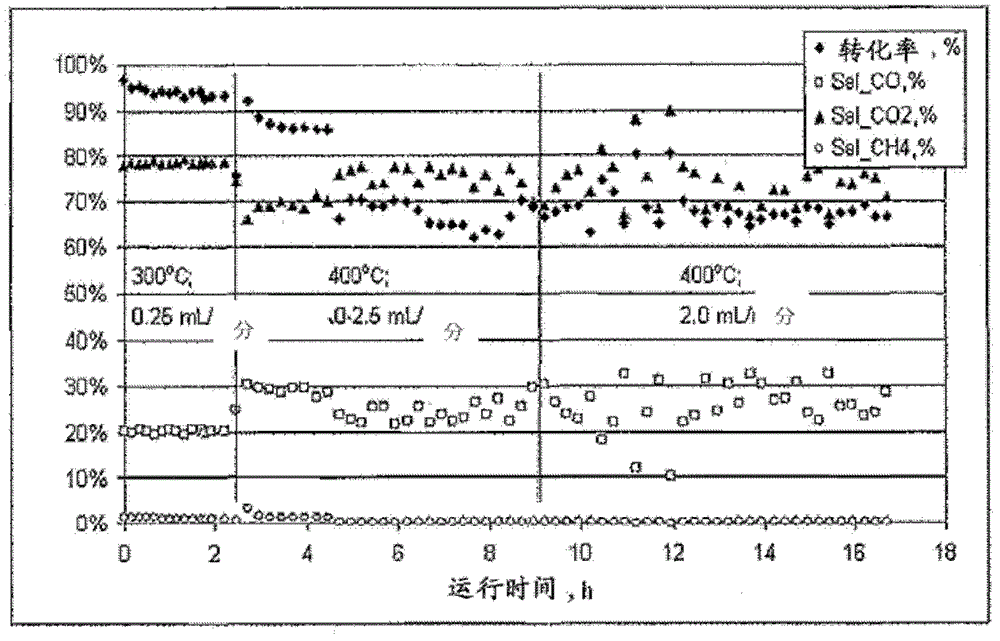
[0054] exist figure 1 As can be seen in , over 95% conversion of methanol was obtained using this catalyst at 300°C with a methanol / water feed rate of 0.25-1.0 mL / min. Increasing the fe...
Embodiment 2
[0055] Example 2: PtTi-Zr / CeO 2
[0056] A first solution containing Pt and Ti was prepared by dissolving 15.244 g of hexahydroxy(IV) platinum acid (H 2 Pt(OH) 6 ) solution (16.40% by weight Pt), 7.611g titanium diisopropoxide bis(acetylacetonate) (C 6 h 28 OeTi, 75% solution in isopropanol) and 1,484 g Zr(OH) 4 Add 35g of water and stir.
[0057] By mixing 7.5g urea and 0.461g Na 2 CO 3 A second solution was prepared by adding 30.0 g of water. The second solution was then mixed with 50.0 g of CeO in a ball mill bottle 2 (HSA20 from Rhodia), resulting in the formation of a dense slurry. The first solution is then contacted with a dense slurry. The resulting mixture was ball milled until about 90% of the solid particles were less than 10 [mu]m in diameter. Subsequently, the slurry was dried at about 120° C. for about 2 h and calcined at about 550° C. in air for about 4 h.
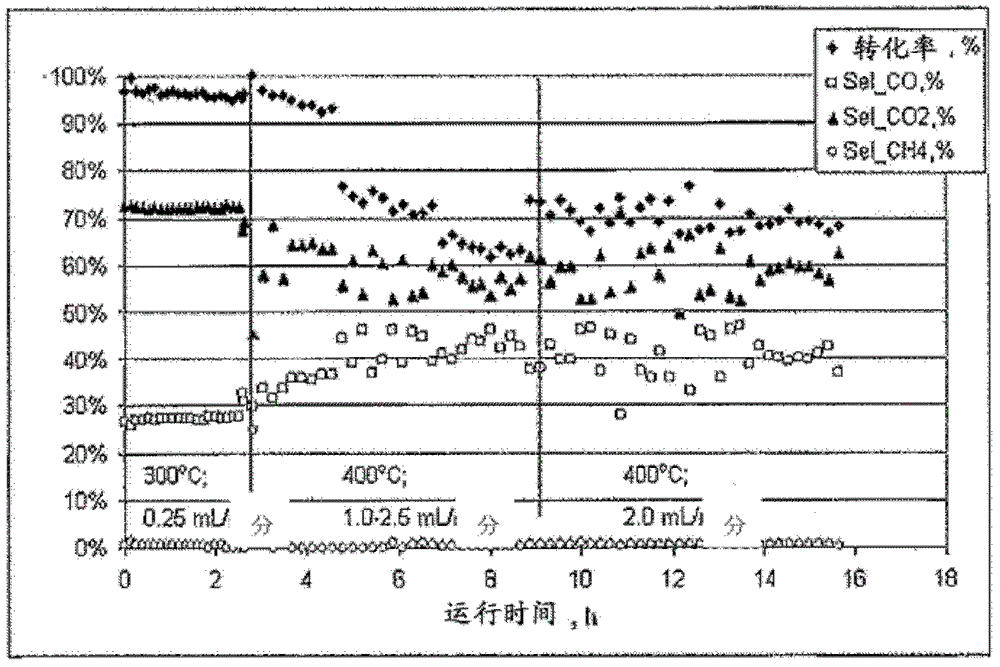
[0058] The performance of the catalyst described above is slightly worse than that of Example...
Embodiment 3
[0059] Embodiment 3: PdGa-ZrY / CeO 2
[0060]A first solution containing Pd and Ga was prepared in the following manner: 11.844 g of Pd(NO 3 ) 2 Solution (20.77 wt% Pd), 27.506 g gallium nitrate hydrate, 1.484 g Zr(OH) 4 and 8.952gY (NO 3 ) 3 ·6H 2 O was added to 42.0 g of water and stirred.
[0061] By mixing 7.5g urea and 0.461g Na 2 CO 3 A second solution was prepared by adding 30.0 g of water. The second solution was then mixed with 50.0 g of CeO in a ball mill bottle 2 (HSA20 from Rhodia), resulting in the formation of a dense slurry. The first solution is then contacted with a dense slurry. The resulting mixture was ball milled until about 90% of the solid particles were less than about 10 μm in diameter. Subsequently, the slurry was dried at about 120° C. for about 2 h and calcined at about 550° C. in air for about 4 h.
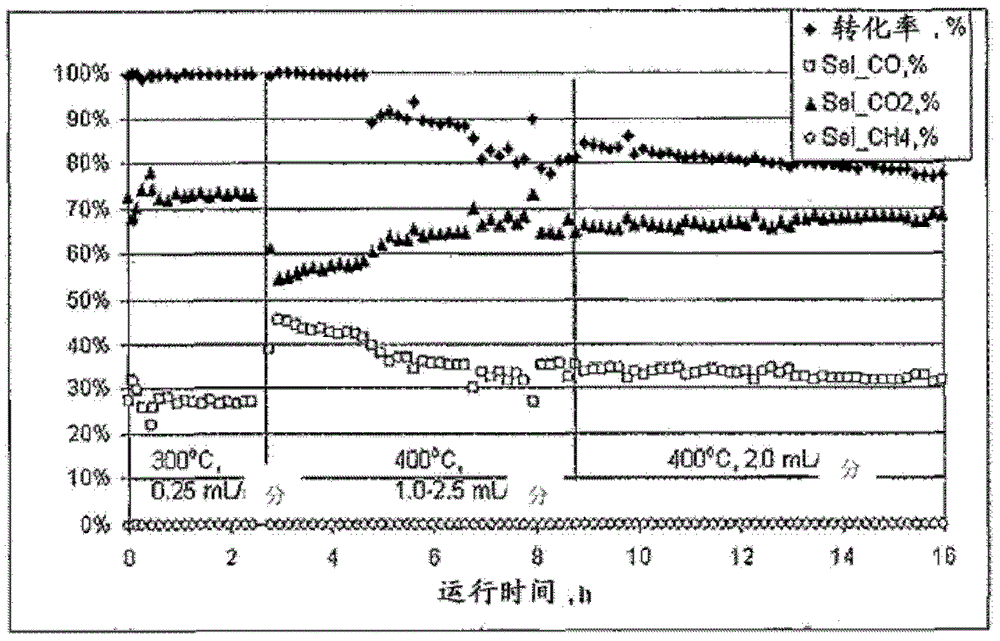
[0062] Complete conversion of methanol was observed at about 300°C with the catalyst of this example using a methanol / water feed rate of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com