Method for preparing cyclopentanone and/or cyclopentanol by furfural or furfuryl alcohol
A technology for cyclopentanone and cyclopentanol, which is applied in the field of preparing cyclopentanone and/or cyclopentanol, can solve problems such as harsh reaction conditions, and achieve the effects of no reduction in activity, simple process, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
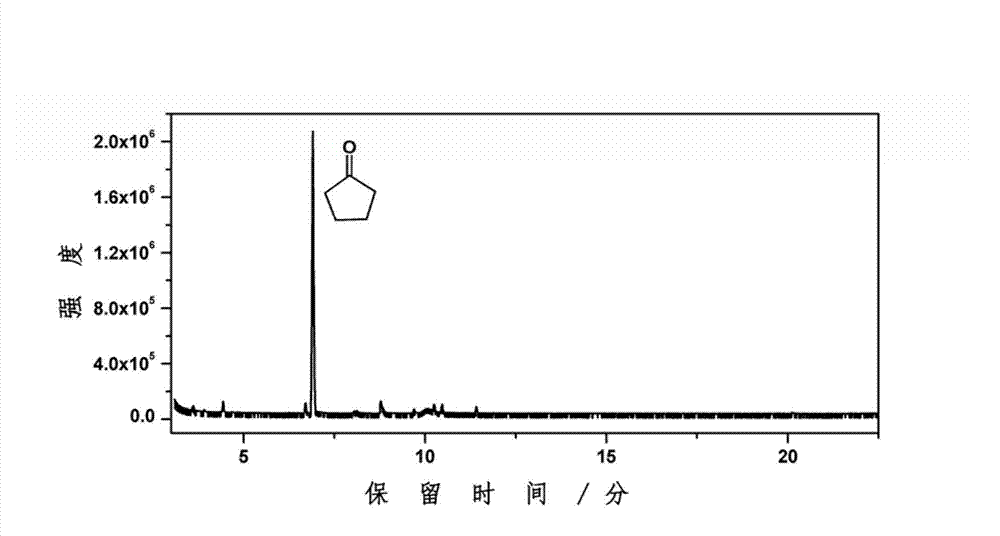
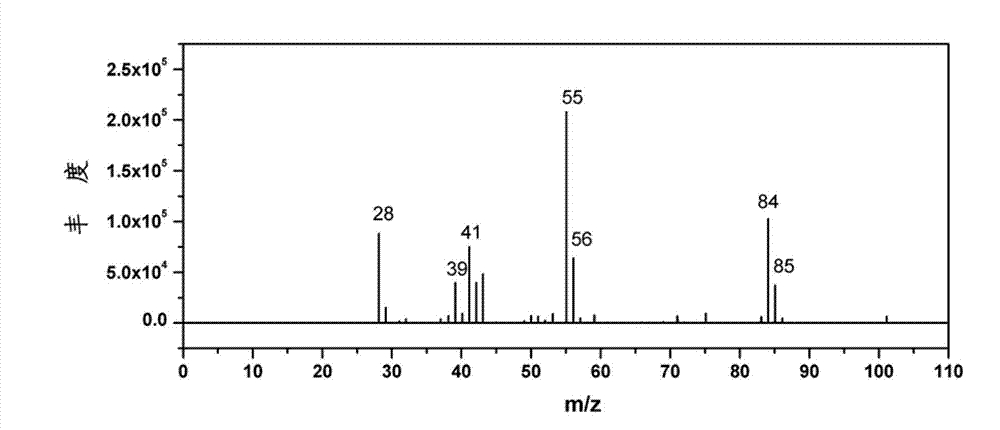
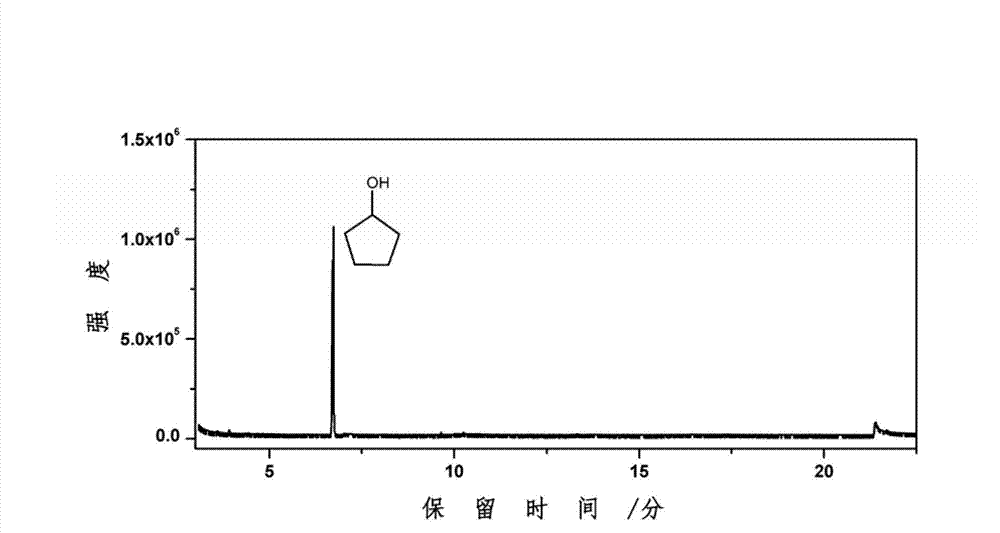
[0027] Weigh 1g of furfural and 20mL of water and put them into a 50mL reactor, and then put in 0.5g of catalyst (CuRu / C). Replace the air in the kettle with hydrogen for three to four times, and then fill it with 14MPa hydrogen to minimize the air content in the kettle, especially the oxygen content, so as to maintain the reducing atmosphere in the kettle. Turn on the stirring device to about 1000 rpm, then heat to 150° C. and maintain it for 4 hours. After the reaction was completed, it was cooled to room temperature, and the liquid product was collected. The liquid product was analyzed for its chemical composition by GC-MS and GC. As a result, the conversion rate of furfural was 98.5%, the yield rate of cyclopentanone was 47.9%, the yield rate of cyclopentanol was 1.4%, and the yield rate of tetrahydrofurfuryl alcohol was 0.8%.
Embodiment 2
[0029] Weigh 1g of furfural and 20mL of water and put them into a 50mL reactor, and then put in 0.5g of catalyst (CuPd / C). Replace the air in the kettle with hydrogen for three to four times, and then fill it with 10MPa hydrogen to minimize the air content in the kettle, especially the oxygen content, so as to maintain the reducing atmosphere in the kettle. Turn on the stirring device to about 1000 rpm, then heat to 200°C and maintain for 4 hours. After the reaction was completed, it was cooled to room temperature, and the liquid product was collected. The liquid product was analyzed for its chemical composition by GC-MS and GC. As a result, the conversion rate of furfural was 100%, the yield rate of cyclopentanone was 24.6%, the yield rate of cyclopentanol was 5.1%, and the yield rate of tetrahydrofurfuryl alcohol was 25.2%.
Embodiment 3
[0031] Weigh 1g of furfural and 20mL of water and put them into a 50mL reactor, and then put in 0.2g of catalyst (CuZnCeAl). Replace the air in the kettle with hydrogen for three to four times, and then fill it with 8MPa hydrogen to minimize the air content in the kettle, especially the oxygen content, so as to maintain the reducing atmosphere in the kettle. Turn on the stirring device to about 1000 rpm, then heat to 150° C. and maintain it for 6 hours. After the reaction was completed, it was cooled to room temperature, and the liquid product was collected. The liquid product was analyzed for its chemical composition by GC-MS and GC. As a result, the conversion rate of furfural was 97.5%, the yield rate of cyclopentanone was 26.5%, the yield rate of cyclopentanol was 1.5%, and the yield rate of tetrahydrofurfuryl alcohol was 47.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com