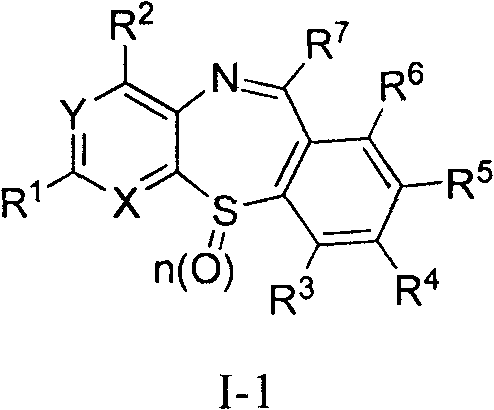
Novel pyrido sulfur nitrogen 7-membered ring derivatives adopted as anti-tumor drugs, preparation method and applications thereof
An anti-tumor drug, drug technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem of drug resistance of anti-cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
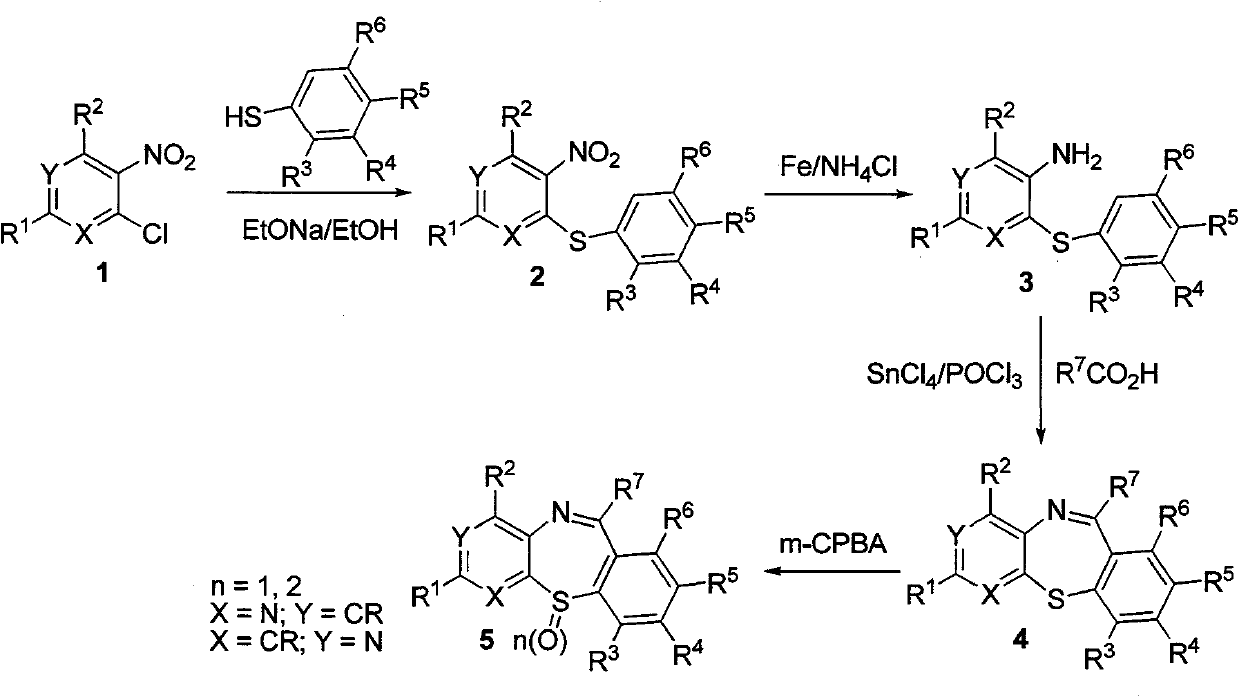
[0208] Example 1: Synthesis of Multi-Substituted Pyridothiazapine Seven-membered Ring Derivatives
[0209] Experimental route:
[0210]
[0211] Synthesis of nitrophenylthiopyridine derivative 2:
[0212]
[0213] Add 40mL of absolute ethanol and sodium (0.92g, 40mmol) to a 100mL single-necked flask, and stir magnetically. After the sodium has reacted, cool in an ice-water bath, add thiophenol (40mmol), and after it dissolves, add chlorinated Nitropyridine 1 (40 mmol), reacted for 20 minutes after the addition, filtered with suction, washed with water, and dried to obtain product 2.
[0214] 2-(4-Methoxyphenylthio)-3-nitropyridine 2.1: mp: 140-142°C; 98%; 1 H NMR (CDCl 3 )δ8.52-8.47 (m, 2H), 7.47 (d, J=9.0, 2H), 7.19-7.14 (m, 1H), 6.98 (d, J=9.0, 2H), 3.86 (s, 3H); ES-MS m / z 263.0[M+H + ].
[0215] 2-(3-Methoxyphenylthio)-3-nitropyridine 2.2: mp: 84-85°C; 94%; 1 H NMR (CDCl 3 )δ8.66-8.39 (m, 2H), 7.41 (q, J=7.5, 1H), 7.18 (dd, J=7.6, 7.2, 1H), 7.14-7.00 (m, 1H), ...
Embodiment 2
[0294] Example 2: Selective cytotoxicity analysis of compounds in human lung cancer, breast cancer, colon cancer, acute leukemia cells and normal fibroblast models
[0295] Cells used in the experiment: human non-small cell lung cancer cells H460, paclitaxel-resistant human non-small cell lung cancer cells H460 TaxR; human breast cancer cell MCF-7 / ADR resistant to doxorubicin; human colon cancer cell HT29; human leukemia cell OP-1; human normal fibroblast NHFB.
[0296] Experimental method: sulforhodamine B (SRB) method (Vnicha Vichail & Kanyawim Kirtikara. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols 1, 2006, 1112-1116.)
[0297] Take H460 cells and H460 cells in the logarithmic growth phase TaxR cells, HT-29 cells, MCF-7 / ADR cells, OP-1 cells and NHFB cells, prepared into 3×10 4 ·mL -1 Add 100 μL of cell suspension to each well and inoculate in a 96-well plate in 5% CO 2 , cultured at 37°C for 24 hours, removed the supernatant, and add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com