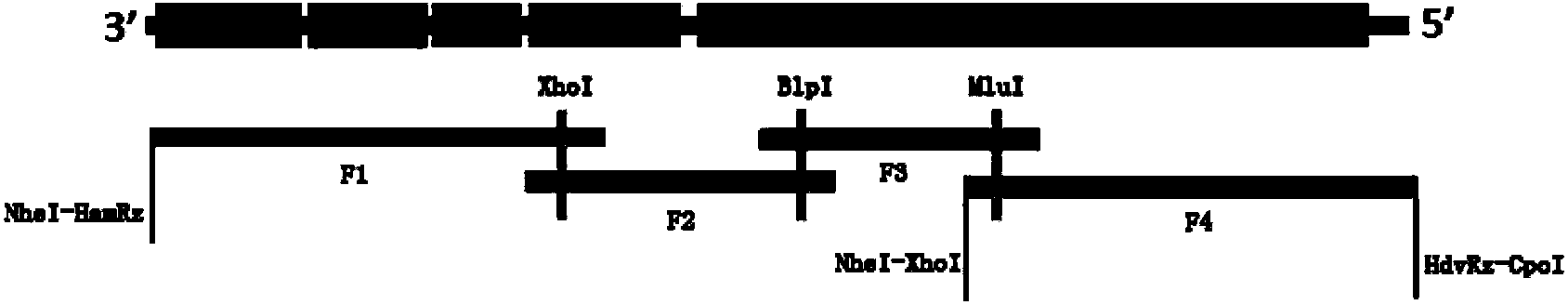Preparation method of recombination live vector vaccines for diseases of canid and/or feline
A live vector vaccine, feline technology, applied in the field of preparation of recombinant live vector vaccines for canine and/or feline epidemic diseases, can solve the problems of lack of international competitiveness, few new vaccine varieties, backward prevention and control technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Construction of recombinant rabies virus eukaryotic expression vector expressing foreign genes
[0031] 1 Materials and methods
[0032] 1.1 Plasmids, cell lines, strains and reagents
[0033] Plasmid pCI ( Figure 11 ), pCDNA3.1(+) ( Figure 12 ), rabies virus SRV9 strain, and BSR cells were purchased from the Institute of Military Veterinary Medicine, Academy of Military Medical Sciences, Chinese People's Liberation Army. BSR cells were cultured in DMEM containing 5% fetal bovine serum, rabies virus SRV9 strain was amplified on BSR cells, and frozen at -70°C for use.
[0034]Phusion DNA polymerase, T4 DNA ligase, and restriction endonuclease were purchased from NEB Company, competent cells were purchased from Takata Company, gel recovery kit and plasmid extraction kit were purchased from Axygen Company, TRIzol, mouse-derived RNA was extracted Reverse transcriptase and liposomes were purchased from Invitrogen Company, and fetal bovine serum was purchased ...
Embodiment 2
[0068] Example 2 Vector construction and identification of recombinant rabies virus expressing eGFP
[0069] 1 Materials and methods
[0070] 1.1 Plasmids, cell lines, strains and reagents
[0071] Plasmids pD-SRV9-PMIn and pD-SRV9-sPMIn were constructed in Example 1, and the plasmid pCI-eGFP containing eGFP ( Figure 13 ) is preserved by the Military Veterinary Research Institute of the Academy of Military Medical Sciences of the Chinese People's Liberation Army.
[0072] 1.2 Construction of expression eGFP vector
[0073] According to the sequence of eGFP, the eGFP fragment with restriction sites at both ends was obtained by PCR method (see Table 4 for PCR primer sequences), and eGFP was inserted into pCI-SRV9-PMin and pCI-SRV9- Among sPMin, pCI-SRV9-PM-eGFP and pCI-SRV9-sPMin-eGFP were obtained. After double digestion with NheI+XhoI, it was ligated into pCDNA3.1(+) vector and named as pD-SRV9-PM-eGFP and pD-SRV9-sPM-eGFP respectively.
[0074] Table 4 Primer sequences ...
Embodiment 3
[0090] Example 3 Construction and identification of recombinant rabies virus vector expressing canine parvovirus VP2 gene
[0091] 1 Materials and methods
[0092] 1.1 Plasmids, cell lines, strains and reagents
[0093] Plasmid pD-SRV9-PM-eGFP was constructed in Example 2, and canine parvovirus CPV (strain CR86106) was purchased from Institute of Military Veterinary Medicine, Academy of Military Medical Sciences.
[0094] 1.2 DNA extraction and PCR
[0095] Extract parvovirus DNA according to the kit instructions.
[0096] 1.3 Construction of recombinant virus expressing parvovirus VP2
[0097] According to the sequence of parvovirus VP2, a VP2 fragment with restriction sites at both ends was obtained by PCR method (see Table 5 for PCR primer sequences), and VP2 was inserted into pD-SRV9-PM-eGFP by BsiWI+PmeI double restriction digestion. pD-SRV9-VP2 was obtained.
[0098] Table 5 Primer sequences
[0099]
[0100] Note: Bold is protective base
[0101] 1.4 Virus res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com