Inhalable Formulations
A technology of excipients and active agents, applied in the field of inhalable preparations, can solve the problem of uneven dosage of content isolation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
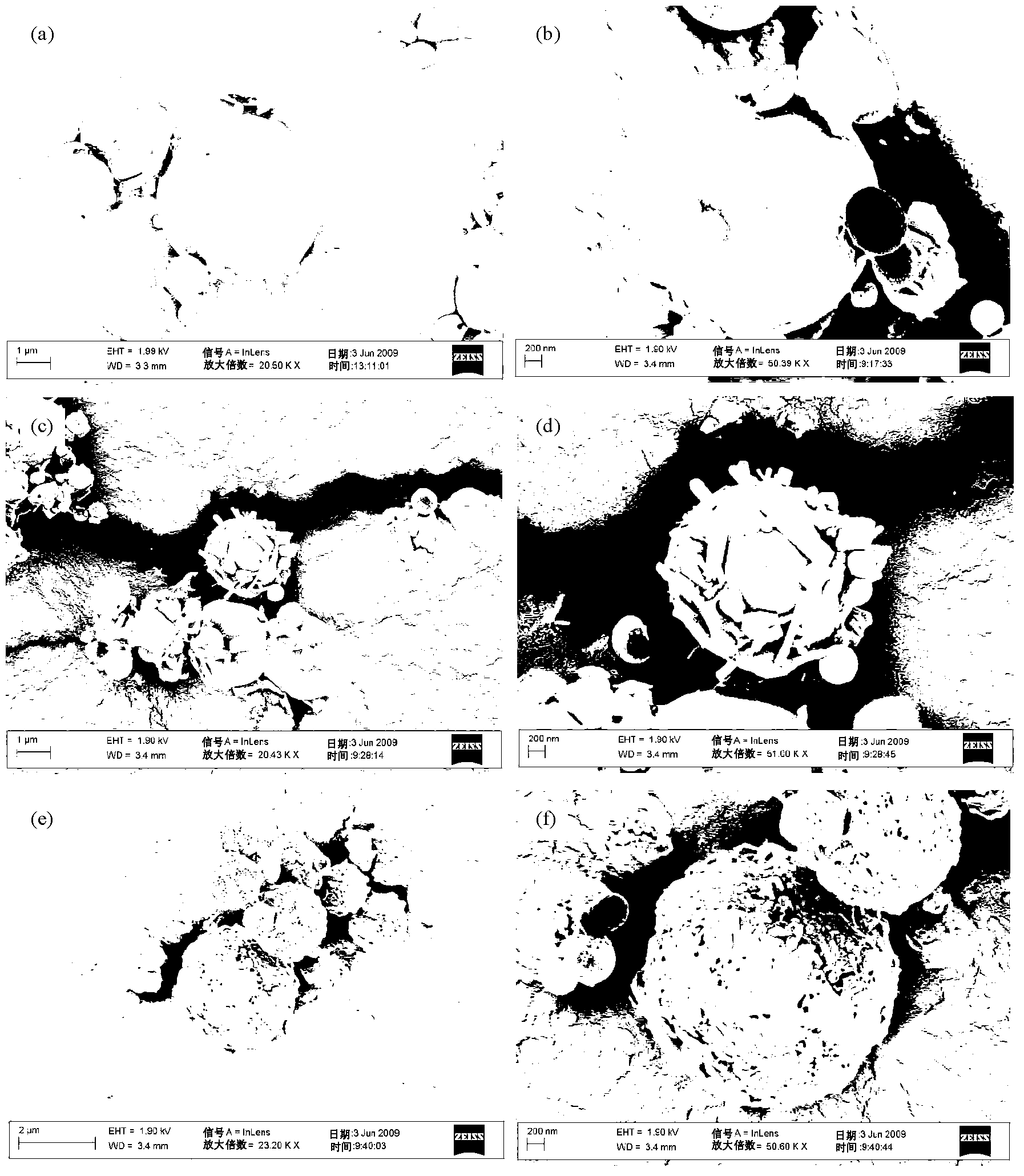
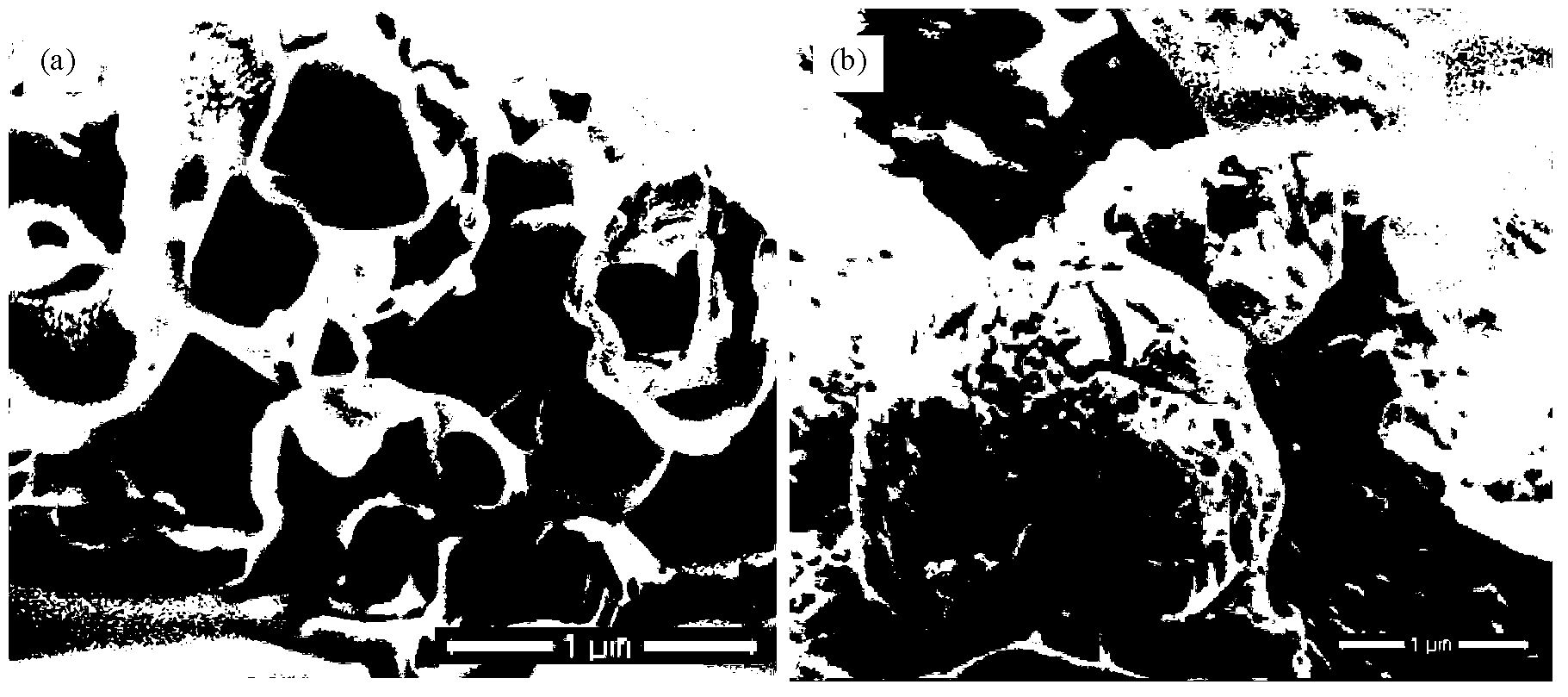
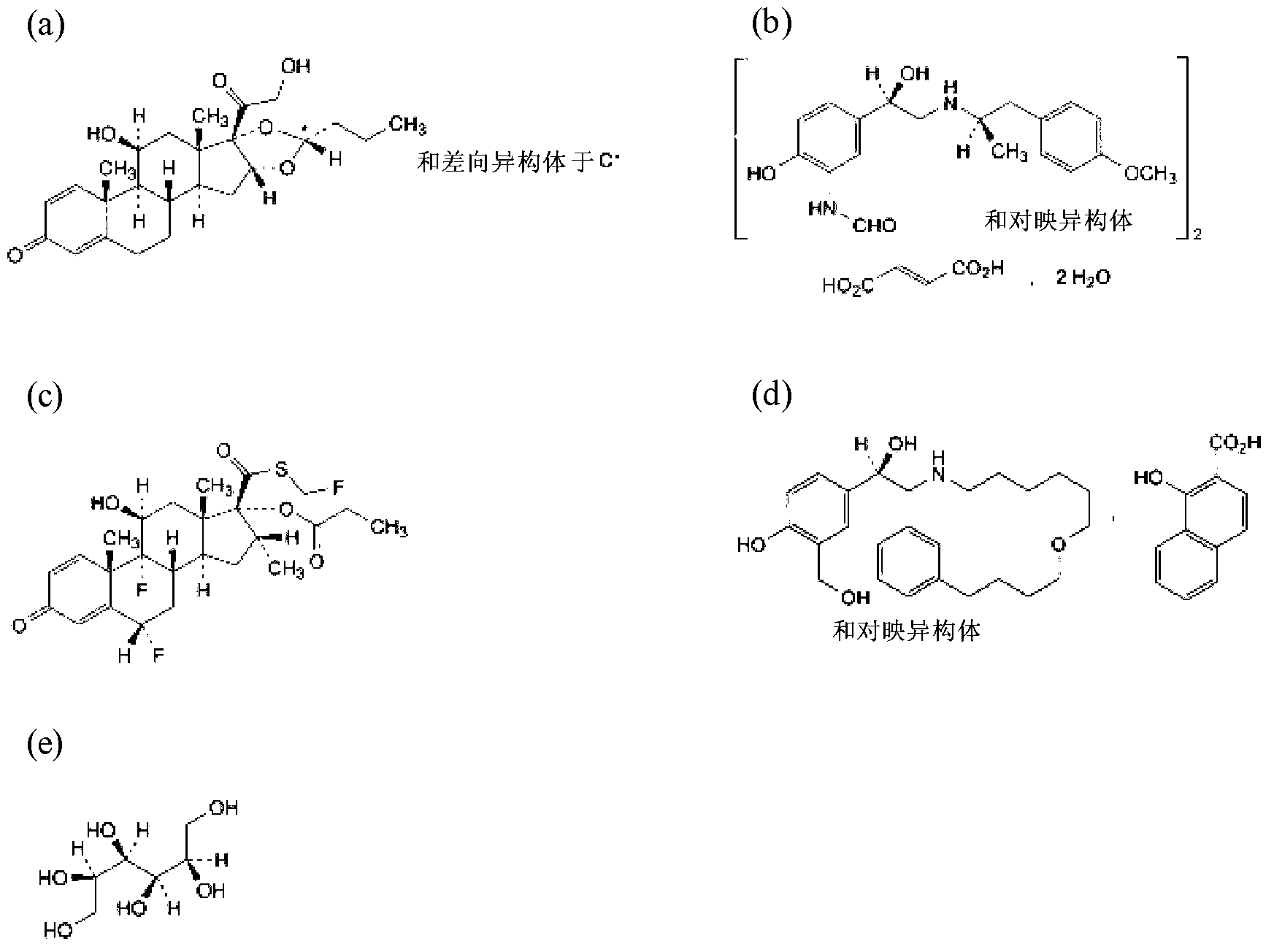
[0174] The following description describes the method of forming particles of the invention from mannitol and an active agent selected from budesonide / fluticasone propionate and formoterol fumarate dihydrate / salmeterol xinafoate. It should be understood that other ingredients, including other active agents and / or other excipients, such as other sugars, can be used to form suitable carrier particles.
[0175] The particles formed herein can be used to form compositions suitable for inhalation by patients in need of such medicaments. This drug can be indicated for the treatment of respiratory conditions such as cystic fibrosis, COPD, bronchitis, allergies, nasopharynx and asthma. It should also be understood that such drugs can be adapted for use in the treatment of non-pulmonary conditions via pulmonary delivery.
[0176] Respirable Particles - Aerodynamic Diameter
[0177] The following information envisions demonstrating how aerodynamic diameter can be determined. This p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com