Method for producing carbonyl fluoride and hexafluoropropylene oxide
A technology of hexafluoropropylene oxide and carbonyl fluoride, which can be used in chemical instruments and methods, carbon compounds, organic chemistry, etc., and can solve problems such as invisible uses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1)
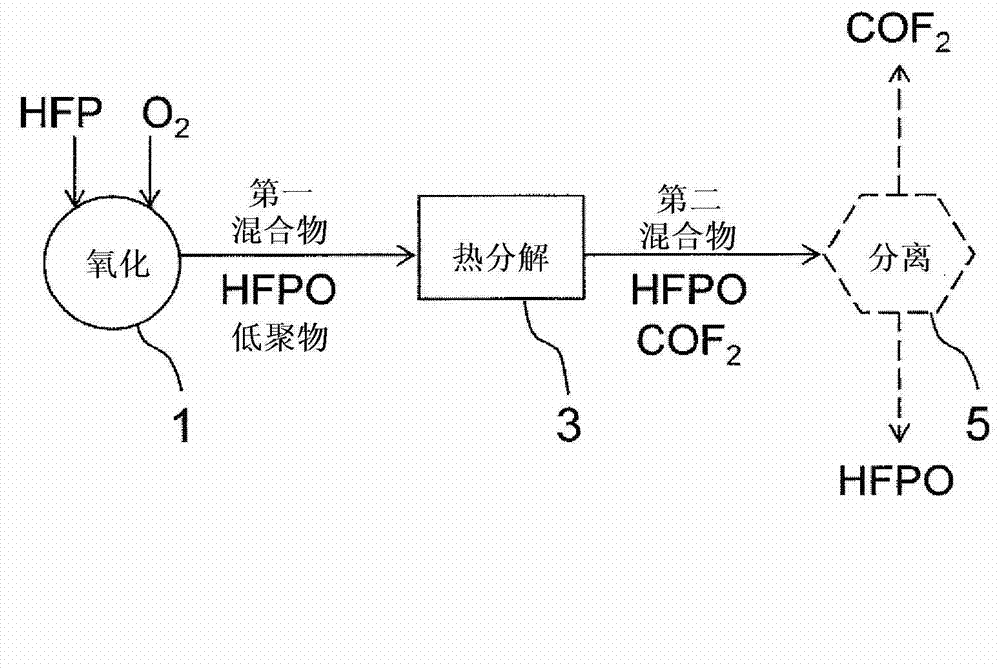
[0044] edge reference figure 1 One embodiment of the present invention will be described in detail.
[0045] ·Process a)
[0046] Such as figure 1 As shown, HFP and oxygen (O 2 ), in reactor 1, HFP is oxidized by oxygen (liquid phase reaction) to generate HFPO.
[0047]
[0048] As the solvent, saturated halogenated hydrocarbons inactive in the oxidation reaction can be used, for example, 1,1,2-trichloro-1,2,2-trifluoroethane, trichlorofluoromethane, perfluoro(dimethyl cyclobutane), carbon tetrachloride, etc.
[0049] In the above oxidation reaction, in addition to HFPO as the target substance, the following general formula (X) is also by-produced:
[0050] CF 3 O(CF 2 O) n -R...(X)
[0051] (In the formula, -R represents -COF, -OCOF or -CF 2 COF, n represents an integer of 0-50, preferably an integer of 0-15. ) of the oligomers shown.
[0052] The oligomer represented by the general formula (X) may be one type of compound, but usually may be a mixture in which a ...
Embodiment approach 2)
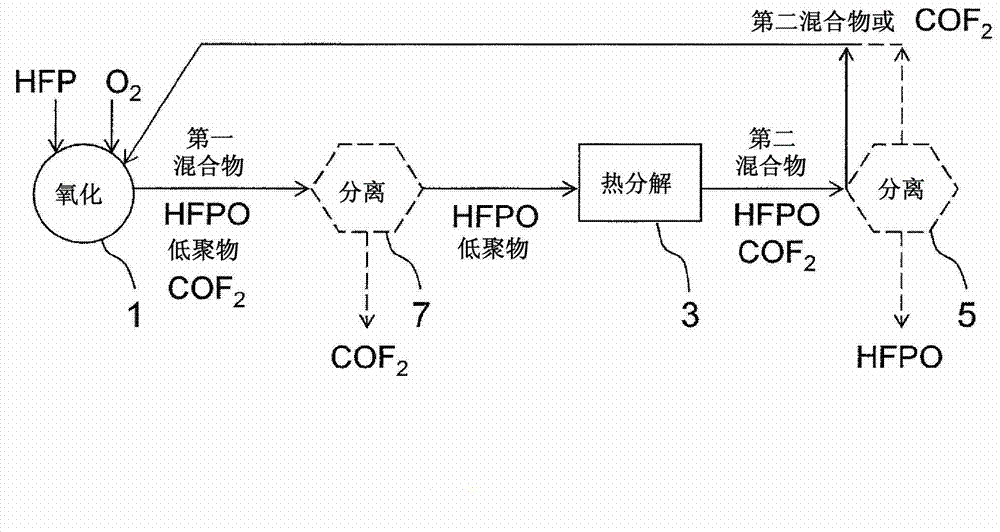
[0081] edge reference figure 2 Another embodiment of the present invention will be described in detail. Hereinafter, the difference from Embodiment 1 will be mainly described, and unless otherwise specified, it is assumed to be the same as Embodiment 1.
[0082] Such as figure 2 As shown, in this embodiment, the second mixture or separated from the second mixture comprising COF 2 The components are returned to Reactor 1. The resulting first mixture, compared to the first mixture in Embodiment 1, COF 2 content (or content rate) increases.
[0083] Generally speaking, because carbonyl fluoride (COF 2 ), so the existing HFPO manufacturing equipment usually includes the separation and recovery of COF 2 s installation. By adding a thermal decomposer 3 to the existing HFPO manufacturing equipment, the COF generated in the thermal decomposer 3 is decomposed 2 Take the second mixture or contain COF 2 The components are returned to the reactor 1, and the COF can be easily se...
Embodiment approach 3)
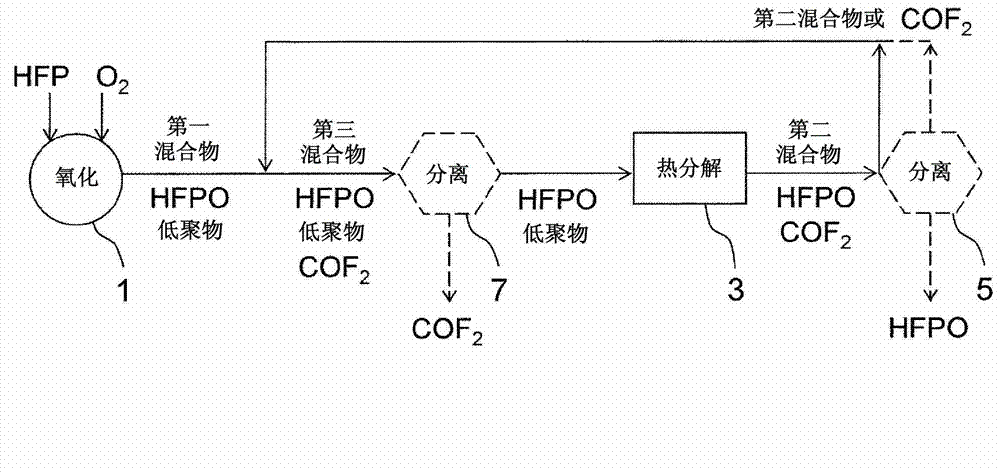
[0087] edge reference image 3 Another embodiment of the present invention will be described in detail. Hereinafter, the difference from Embodiment 2 will be mainly described, and it is the same as Embodiment 2 unless otherwise specified.
[0088] Such as image 3 As shown, in this embodiment, between the reactor 1 and the thermal decomposer 3, the second mixture is added to the first mixture or the COF-containing 2 components as the third mixture. Compared to the first mixture, the resulting COF of the third mixture 2 The content (or content rate) increases.
[0089] As mentioned above, the existing HFPO manufacturing equipment usually includes the separation and recovery of COF 2 s installation. By adding a thermal decomposer 3 to the existing HFPO manufacturing equipment, the COF generated in the thermal decomposer 3 is decomposed 2 , to a second mixture or containing COF 2 By way of components, returning to any suitable position between reactor 1 and thermal decomp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com