Application of chitosan as adjuvant in preparation of influenza virus attenuated live vaccine
A technology for live attenuated vaccines and influenza viruses, which can be used in antiviral agents, medical preparations containing active ingredients, medical components of antibodies, etc. The effect of expanding the immunized population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] One: the preparation of chitosan solution:
[0024] Weigh 3.38g of sodium glutamate and dissolve it in 100ml of water to prepare a 0.2mol sodium glutamate solution. The pH of the solution was adjusted to 2-3 with acetic acid. Weigh 0.4g chitosan into the solution and stir until completely dissolved. The pH value of the solution was adjusted to 4-5 with sodium hydroxide, sterilized by filtration with a 0.22 nm filter membrane, and stored at 4°C in aliquots for later use.
[0025] Two: the preparation of influenza virus attenuated live vaccine:
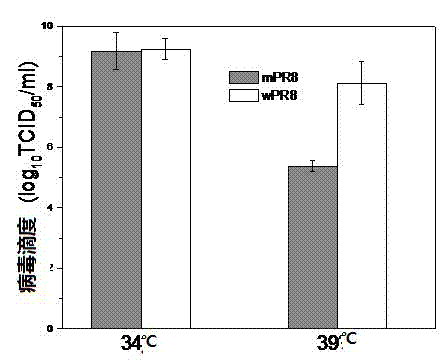
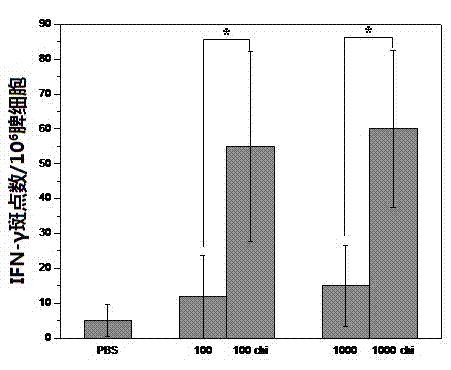
[0026] Extract virus RNA from the allantoic fluid of chicken embryos inoculated with A / Puerto Rico / 8 / 34 (H1N1) virus, use RT-PCR technology to prepare cDNA, and amplify PB2, PB1, PA, HA, NP of influenza virus , NA, M, NS eight gene segments. Mutations were introduced in PB1 (K391E, E581G, A661T) and PB2 (N265S) by overlapping PCR. BbM I digestion PCR fragment and Phw2000 plasmid, T4 DNA ligase connection. With the help of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com