Combined use of cry1da and cry1fa proteins for insect resistance management
An insect and protein technology, applied in the field of compositions to control Lepidoptera pests, can solve problems such as limited research capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
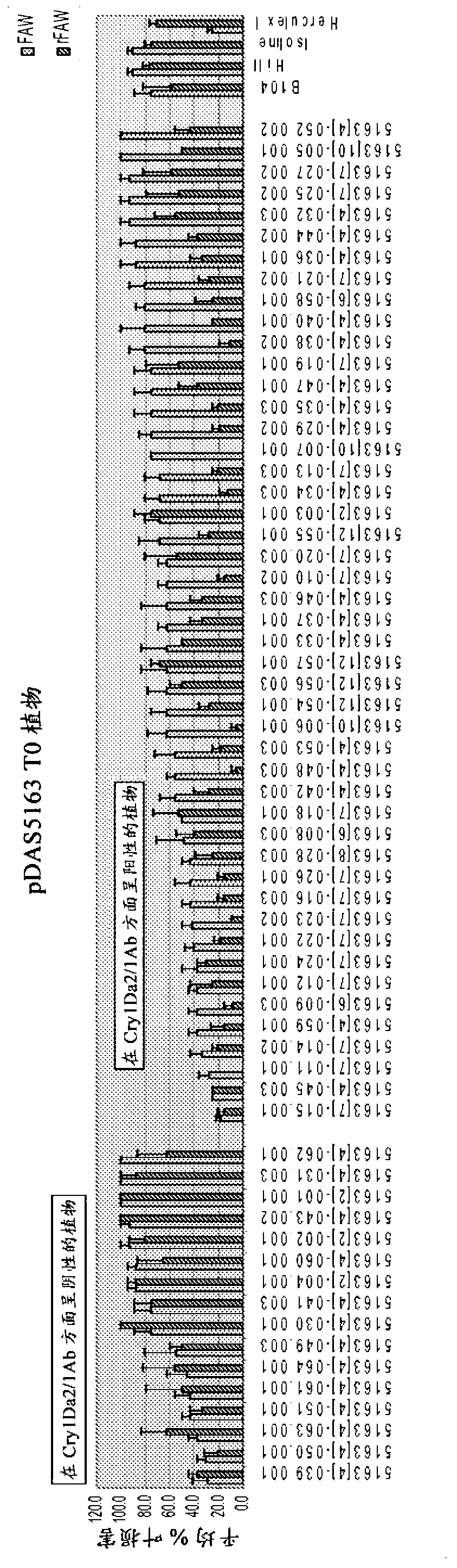
[0092] biometric data
[0093] Cry1Da expressed in transgenic maize (pDAS5163) confers protection from Fall Armyworm (FAW) Spodoptera frugiperda (J.E. Smith). The same event was more effective in controlling FAW that had developed resistance to CrylFa, and significantly outperformed maize plants containing event TC1507, which is arguably an industry-leading insect resistance trait for FAW control.
[0094] We have also demonstrated that both Cry1Fa (protein from recombinant Pseudomonas fluorescens strain DR1649; plasmid pDAB1817) and Cry1Da (protein from recombinant Pseudomonas fluorescens strain DC782) effectively control FAW in artificial diet bioassays, and The potency of the combination was greater than expected from their individual potencies.
[0095] Based on the data described above, co-expression of CrylDa and CrylFa can generate high doses of IRM stacks against FAW, other important Spodoptera species, and possibly other Lepidopteran pests. Other proteins can be add...
Embodiment 2
[0104] Aggregation of Combined Data
[0105] The following describes the use of 125 Competition binding experiments with I-labeled CrylDa using brush border membrane vesicles (BBMVs) isolated from FAW. Results from these experiments indicate that Cry1Da binds tightly to its receptor and that Cry1Fa does not compete with Cry1Da for the binding site. If resistance to Cry1Da can be based on mutations in the receptor observed in these studies, these data suggest that Cry1Fa would be a good IRM tool for managing such resistant populations or mitigating the development of such resistance. Results from bioassays with CrylFa resistant FAW (rFAW) indicated that CrylDa was active against this population. These data collectively suggest that Cry1Fa and Cry1Da may be an IRM stack effective in alleviating the development of resistance to either insecticidal protein.
[0106] Receptor binding assays showed 125 I Cry1Da binds tightly to its receptor and can be efficiently competed by unl...
Embodiment 3
[0109] Design of Chimeric Toxins Comprising Cry1 Core Toxins and Heterologous Protoxins
[0110] chimeric toxin . Chimeric proteins utilizing the core toxin domain of one Cry toxin fused to a protoxin segment of another Cry toxin have been reported previously, eg, in US Patent No. 5,593,881 and US Patent No. 5,932,209.
[0111] The Cry1Da chimeric protein variants of the invention include chimeric toxins comprising an N-terminal core toxin segment derived from a Cry1Da insecticidal toxin fused thereto within a heterologous delta at a point beyond the end of the core toxin segment Toxin protoxin segment. The transition from the core toxin to the heterologous protoxin segment can occur approximately at the native core toxin / protoxin junction or, in the alternative, a portion of the native protoxin (extending beyond the core toxin segment) can be retained, where in The transition to heterologous protoxin occurs downstream. In variant fusions, the core toxin and protoxin se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com