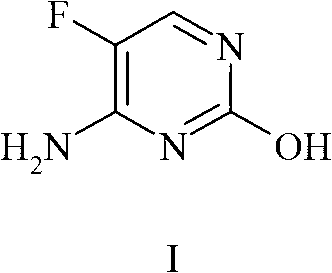
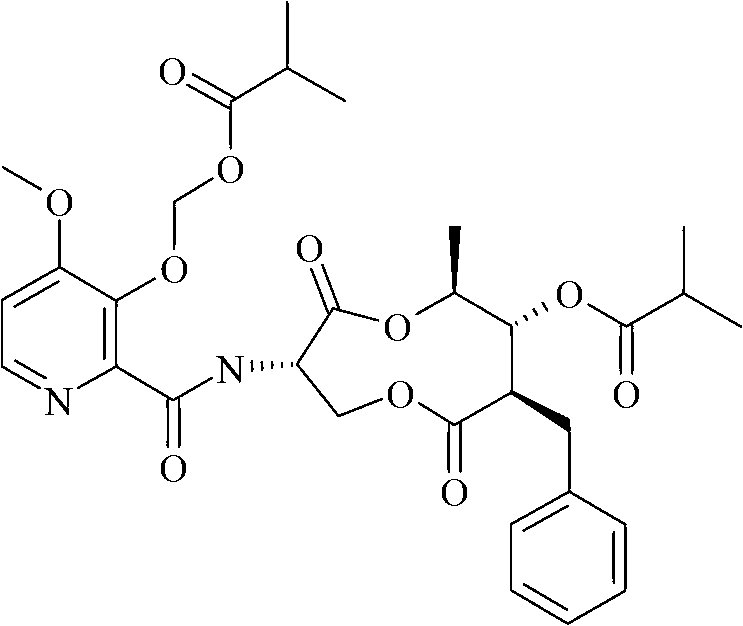
Synergistic fungicidal composition containing 5-fluorocytosine for fungal control in cereals
A synergistic and fungicidal technology, applied in the field of synergistic fungicidal compositions containing 5-fluorocytosine for fungal control in grains, capable of solving problems such as resistance to fungicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Representative organic liquids that may be employed in the preparation of the emulsifiable concentrates of the present invention are aromatic liquids such as xylene and propylbenzene fractions; or mixed naphthalene fractions, mineral oils, substituted aromatic organic liquids such as phthalates Dioctyl formate; kerosene; dialkylamides of various fatty acids, especially dimethylamides of fatty diols and diol derivatives such as n-butyl, ethyl or methyl ethers of diethylene glycol Ether and methyl ether of triethylene glycol, etc. Mixtures of two or more organic liquids are also suitably employed in the preparation of emulsifiable concentrates. Preferred organic liquids are xylene and propylbenzene fractions, with xylene being most preferred. Surface active dispersants are typically employed in liquid formulations and are present in an amount of 0.1 to 20 wt%, based on the combined weight of the surface active dispersant and synergistic composition. The formulations may...
Embodiment
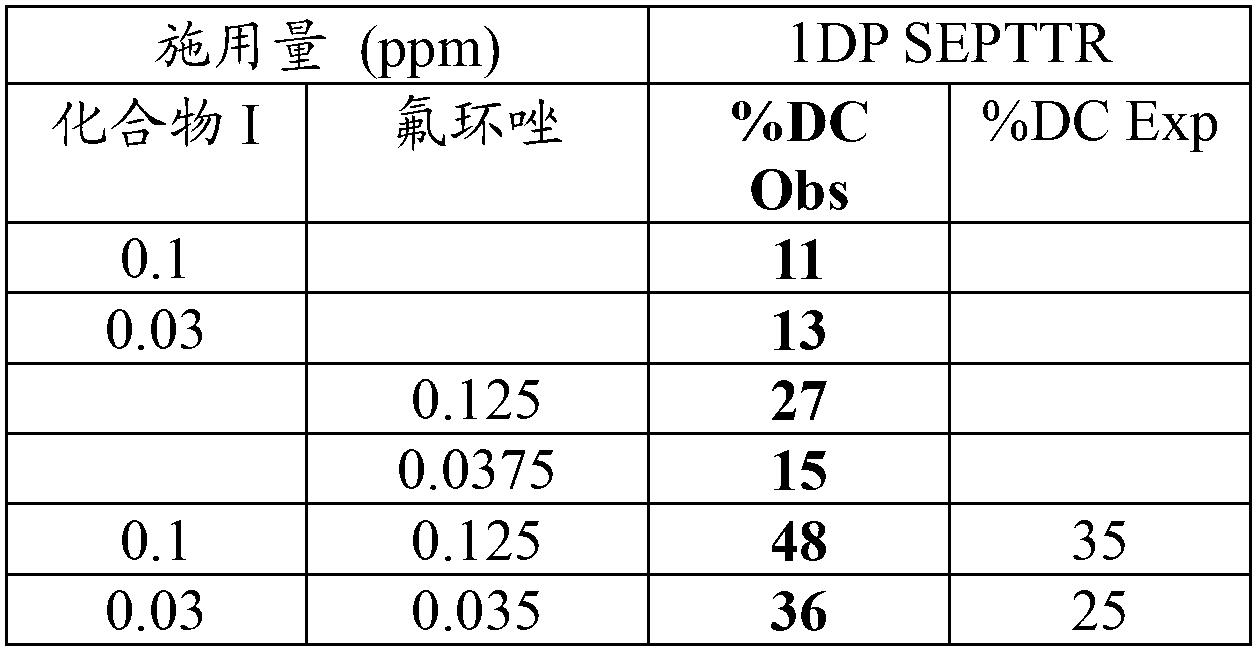
[0047] Fungicide mixture against spot blight of wheat (Gremina graminearum; anamorph: Septoria tritici; Bayer coded as: SEPTTR) evaluation of therapeutic agent and protective agent activity
[0048] Wheat plants (var. Yuma) were grown from seed in a greenhouse in plastic pots with a surface area of 27.5 square centimeters (cm2) containing a 50% mineral soil / 50% soilless Metro mix, 8-12 seeds per pot. The plants were used for testing when the first leaves had fully emerged (this usually required 7 to 8 days after planting). The test plants were inoculated with an aqueous spore suspension of Septoria tritici 3-days before (3-day treatment test; 3DC) or 1-day after (1-day protectant test; 1DP) of fungicide treatment. After inoculation, the plants were kept at 100% relative humidity (1 day in a dark dew chamber, then two days in a lighted mist chamber) to allow spores to germinate and infect the leaves. The plants were then transferred to the greenhouse until the disease ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com