Novel genomic biomarkers for irritable bowel syndrome diagnosis
A technology of irritable bowel syndrome and biomarkers, applied in disease diagnosis, biological testing, genetic engineering, etc., can solve problems such as differential diagnosis of early disease and obstacles to effective treatment, difficulties in rapid and accurate diagnosis, and patient differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] This example describes blood sample collection and RNA isolation therefrom. Briefly, blood samples were collected from 3 IBS-D patients, 2 IBS-C patients and 3 healthy volunteers. All IBS patients met Rome III criteria, and healthy volunteers had no history of IBS or other active comorbidities. In this case, approximately 2.4 ml of whole blood was collected from each subject. Blood samples were divided into 2 aliquots and one was processed according to the leukocyte protocol described above, while the other was collected in the PAXgene system (PreAnalytX; Hombrechtikon, Switzerland) and processed accordingly.
[0243]Since the main difference between these 2 techniques is the inclusion of RNA from the erythrocyte fraction, it was investigated whether the excessively large amount of hemoglobin mRNA could explain the difference in expression between whole blood and leukopoietic samples. Additional RNA was isolated from whole blood from healthy subjects using the PAXgene...
Embodiment 2
[0245] This example describes hybridization of extracted mRNA samples to oligonucleotide arrays. For the analysis of IBD and control serum RNA samples, the Affymetric human gene 1.0ST array (Affymatrix, Santa Clara, CA) was used. These arrays are oligonucleotide probe-based gene array chips containing ~35,000 transcripts, which provide comprehensive coverage of the whole human genome.
[0246] To prepare RNA samples for hybridization, 8 μg of total RNA was used for cDNA synthesis. Following the manufacturer's protocol (Affymatrix, San Diego, California), the T7 promoter sequence introduced during first-strand synthesis was then used to direct cRNA synthesis, which was labeled with biotinylated deoxynucleotide triphosphates. After fragmentation, the biotinylated cRNA was hybridized to the GeneChip array at 45°C for 16 hours. Chips were washed, stained with phycoerythrin-streptavidin, and scanned with Gene Chip Scanner 3000. After background correction, preliminary data analy...
Embodiment 3
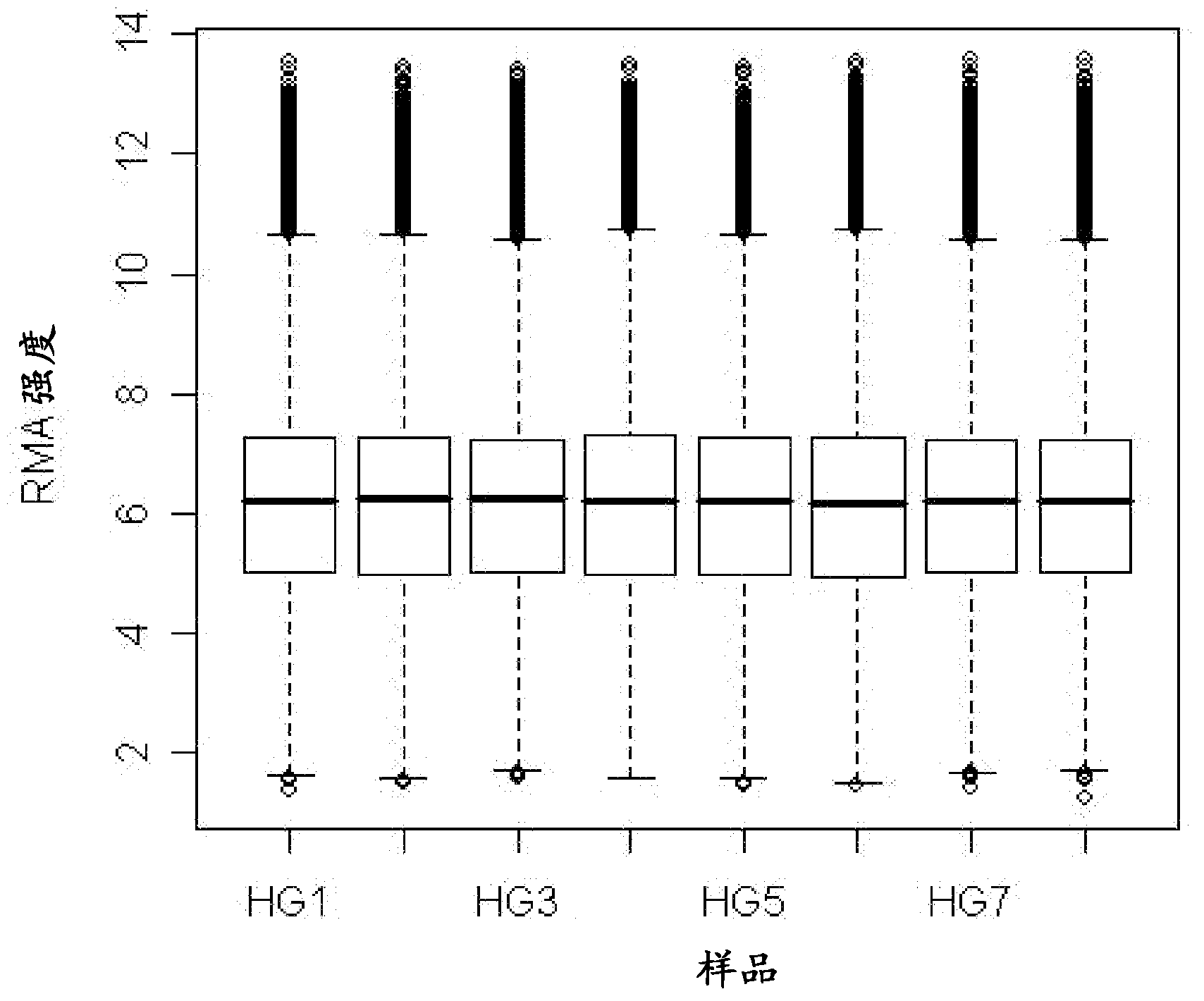
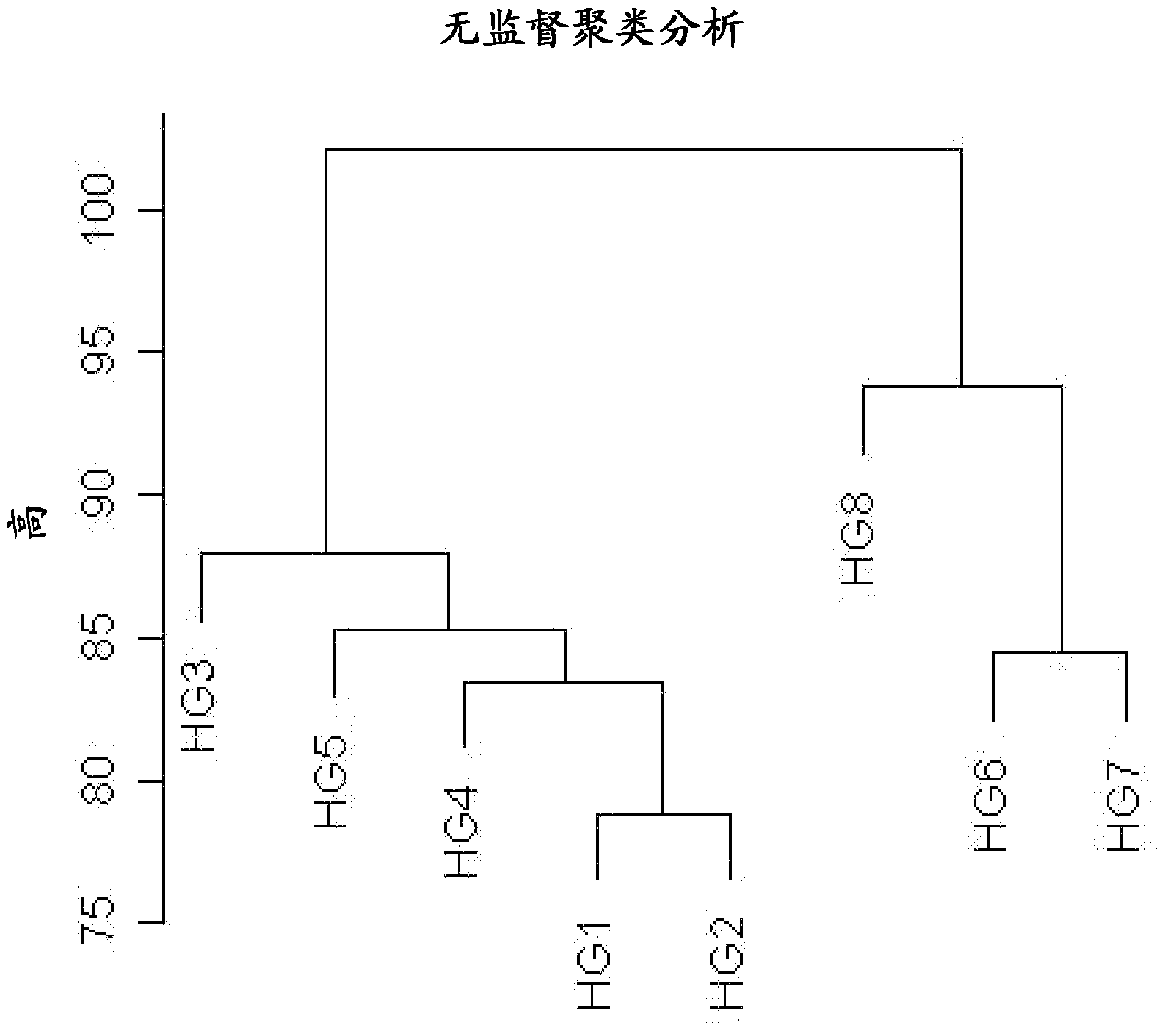
[0248] This example describes data analysis of microarrays performed as described in the previous examples. Analyze the RNA Integrity Number (RIN) of each sample and the performance of each microarray experiment for quality control purposes. The results are given in Table 3.
[0249] Table 3. RNA sample and microarray quality control matrix.
[0250]
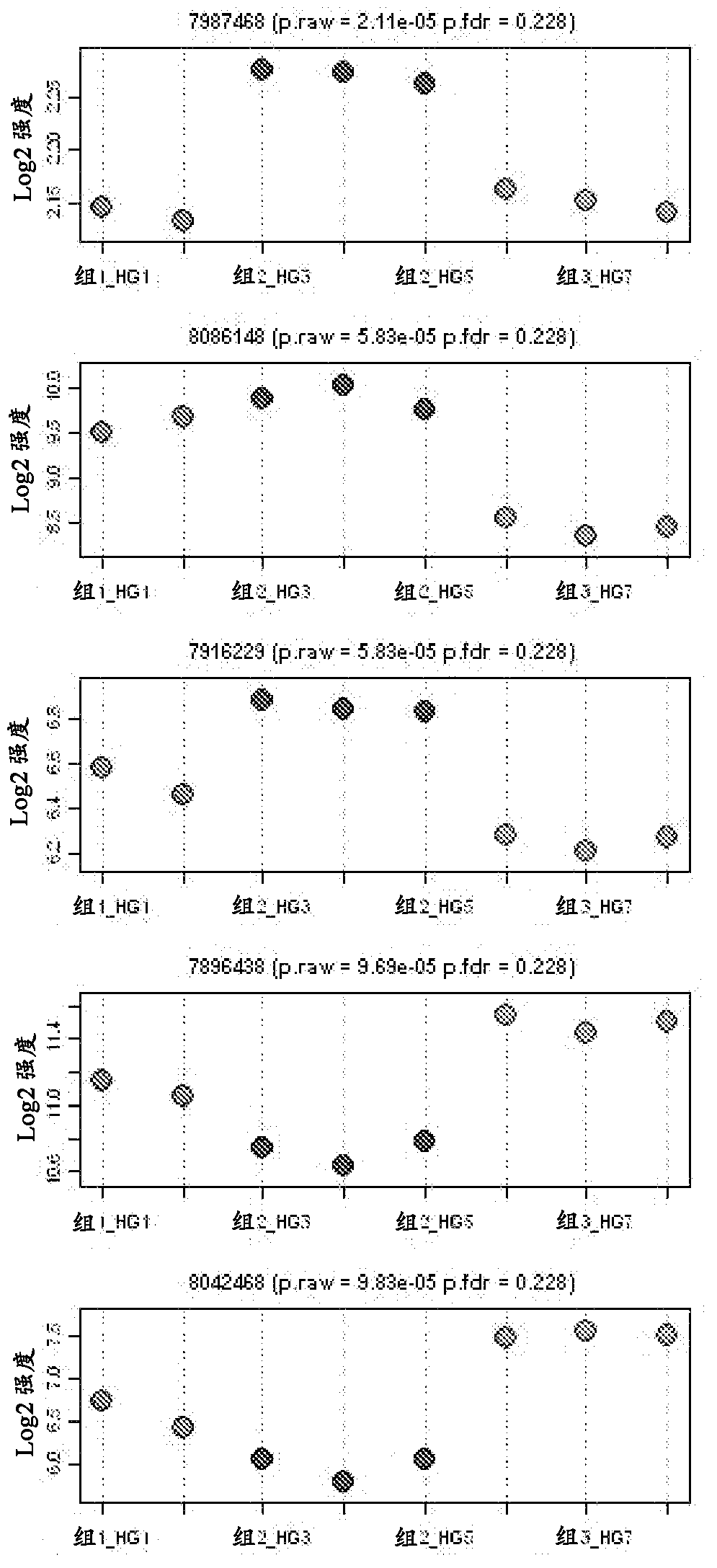
[0251] The fluorescence intensity of each probe set was uploaded to Array Assist 6.5 and Gene Spring GX10.0 (Agilent Technologies, Santa Clara) software. Data were normalized by quantitative normalization and then log-transformed for further analysis to identify changes in specific genes in IBS patients. To compare changes in gene expression, the data were further normalized by using the 50 RFU fluorescence value as a threshold, and statistical analyzes showing fold changes (p ≤ 0.05) were determined. Using the 2 log2 fold change as cutoff, the top 72 markers were identified and are shown in Table 4.
[0252] Table 4. Top...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com