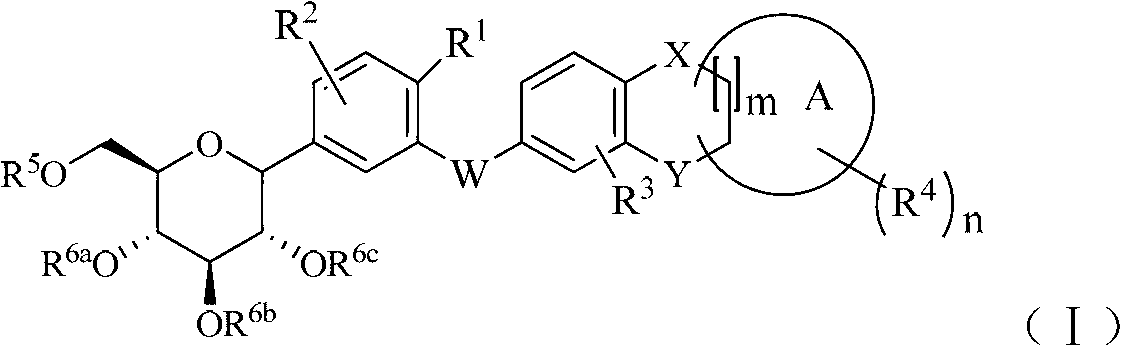
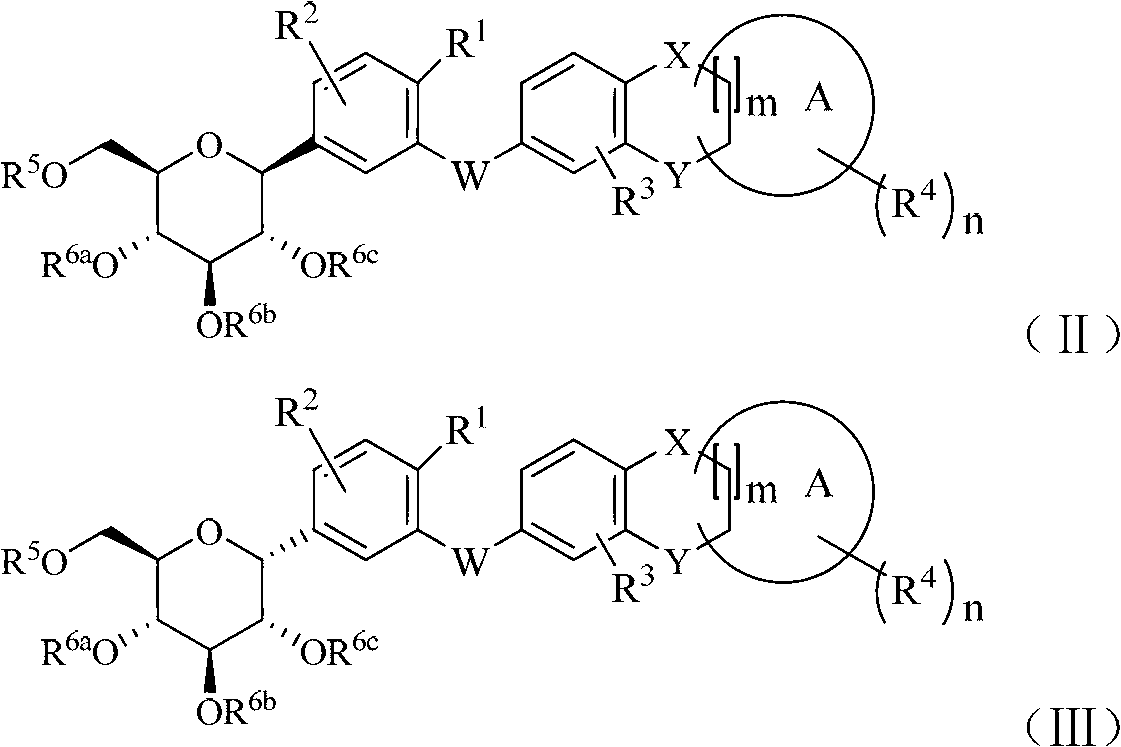
C-indican derivative
A compound and alkyl technology, applied in the field of medicine, can solve problems such as insufficient net energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0115] Step 1 Preparation of compound a
[0116] Dissolve the raw material 1 and N-methylmorpholine in THF, cool under the protection of nitrogen, slowly add trimethyl silicon chloride dropwise, keep the temperature, raise the temperature and stir the reaction after completion, then stir the reaction at room temperature, add toluene to dilute and cool. Water was added to maintain the temperature, the organic phase of the reaction mixture was separated, and washed with aqueous sodium dihydrogen phosphate, water, and saturated brine. Rotary evaporation gave a pale yellow oil a.
[0117] Step 2 Preparation of compound b
[0118] The dichloromethane solution of aluminum trichloride was cooled to 0°C, raw material 3 was slowly added, and the mixture was kept at 0°C for 1 h, and then the dichloromethane solution of raw material 2 was slowly added dropwise. The reaction was detected until the end of the reaction, and the reaction mixture was poured into ice water. It was extracted three t...
Embodiment 1
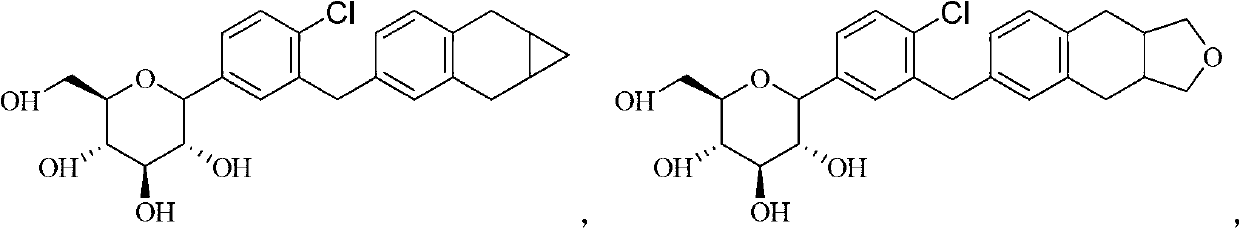
[0163] Example 1 β-1'-deoxy-1'-(4-chloro-3-(1a,2,7,7a-tetrahydro-1H-cyclopropyl[b]naphthalene-4-methylene)benzene) -Glucose acetal (Compound 1) Preparation
[0164]
[0165] Step 1 Preparation of 2,3,4,6-tetra(trimethylsilyl ether)-gluconolactone
[0166]
[0167] Dissolve raw material 1 (239g, 1.34mol) and N-methylmorpholine (1.18L, 10.73mol) in THF (2.4L), cool to -5°C under nitrogen protection, and slowly add trimethyl silicon chloride dropwise (1022mL, 8.05mol), keep the temperature of the dripping process not exceeding 5℃, after completion, raise the temperature to 35℃ and stir for 5h, then stir at room temperature for 15h, add toluene to dilute and cool to 0-5℃, then add water to keep the temperature not exceeding 10 At °C, the organic phase of the reaction mixture was separated and washed with aqueous sodium dihydrogen phosphate, water and saturated brine. By rotary evaporation, 593.2 g of light yellow oily 2,3,4,6-tetrakis(trimethylsilyl ether)-gluconolactone was obtain...
Embodiment 2
[0189] Example 2 β-1'-deoxy-1'-(4-chloro-3-(3,3a,4,9,9a-hexahydronaphthalene[2,3-c]furan-6-methylene)benzene )-Glucose acetal (compound 2) Preparation
[0190]
[0191] Reference Example 1 to obtain the compound β-1'-deoxy-1'-(4-chloro-3-(3,3a,4,9,9a-hexahydronaphthalene[2,3-c]furan-6-methylene Benzene)-glucose acetal. LC-MS(M+H) + :461.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com